Abstract
Background Induction therapy followed by autologous stem cell transplant (ASCT) and maintenance is the current standard of care for the treatment of newly diagnosed multiple myeloma (NDMM). However, indefinite maintenance therapy after ASCT carries both significant clinical and financial toxicities. While minimal residual disease (MRD) testing has become part of routine standard of care, its use in guiding treatment decisions, in particular cessation of therapy, has not been established in the maintenance setting. We hypothesize that MRD monitoring could be used to guide clinical decision making, justifying cessation of maintenance therapy in a sub-group of patients with sustained MRD-negative remission.
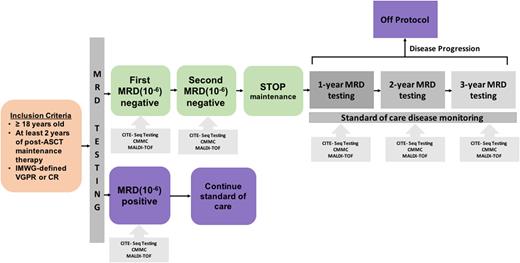
Study Design and Methods This is a phase 2 single-arm adaptive design study (NCT 05192122) for decision-making regarding maintenance therapy (Figure 1). Patients are eligible if they have a diagnosis of active MM, have completed at least 2 years of maintenance therapy post-ASCT, and meet International Myeloma Working Group (IMWG) criteria for very good partial response (VGPR) or complete response (CR). MRD testing is performed twice, at least one year apart, on bone marrow aspirate using next-generation sequencing (NGS) and MRD-negative status is defined at a threshold of 10-6. Patients with negative PET scan in VGPR or CR and sustained (i.e., twice, 1 year apart) MRD negative status discontinue maintenance therapy. MRD-positive patients continue continue maintenance and are taken off study. In patients who are MRD-negative and discontinue maintenance therapy, MRD testing will be re-assessed yearly for 3 years. At each time of MRD testing, MALDI-TOF spectrometry and measurement of circulating multiple myeloma cells (CMMCs) will be done in parallel to compare against MRD results. Health-related quality of life (HRQoL) will be assessed throughout and results on maintenance compared with those after discontinuation using the EORTC QLQ-MY20 questionnaire. This study will enroll 50 patients to address the primary objective of assessment for sustained MRD-negative status at 12 months after stopping maintenance therapy.
For all patients, a 5-mL peripheral blood and bone marrow sample is collected and stored at the time of each standard of care bone marrow biopsy and will be used to characterize the immune microenvironment by Cellular Indexing of Transcriptomes and Epitopes by Sequencing (CITE-seq) of paired peripheral blood and bone marrow in MRD-negative and MRD-positive patients as well as of patients progressing after stopping therapy.
Preliminary Results Enrollment into FREEDMM trial began in December 2021, and to date 20 patients in CR have undergone a first bone marrow for MRD assessment, with results pending in 9 patients. The median age is 66.5 (range: 39-76) years, and the majority of patients are either Black (n=7; 35%) or Hispanic (n=7; 35%). One patient has high risk disease (17p deletion). The median number of years of maintenance therapy completed is 4.2 (range: 2.14-7.9) years. 17 (85%) patients had a negative MALDI-TOF test at the time of the first marrow. Seven (63.6%) of 11 patients were found to be MRD-negative. MALDI-TOF failed to accurately correlate with MRD results in 2 patients. 40 paired peripheral blood (n=20) and bone marrow aspirate (n=20) samples have been collected for CITE-seq analysis. Pilot CITE-seq experiments captured 6,700-11,000 cells with between 38,000-68,000 reads per cell on one MRD-negative and one MRD-positive bone marrow aspirate and paired peripheral blood sample. CITE-seq results for these 20 patients will be presented at the conference.
Conclusion: This phase 2 trial applies a novel clinical trial design with an adaptive strategy to explore use of MRD testing as a guide to discontinuing maintenance therapy in myeloma and represents one of the first studies to explore in parallel various measures of disease monitoring (i.e., MRD, MALDI-TOF, CMMCs, CITE-seq). Ongoing results will be reported at the conference.
Disclosures
Hofmeister:Genzyme: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; BlueBird Bio: Honoraria. Quigley:Agios: Speakers Bureau; Alnylam: Speakers Bureau; Servier: Speakers Bureau; Rigel: Other: Advisory Board. Sborov:Pfizer: Consultancy; Amgen: Consultancy; Bioline: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy; Sanofi: Consultancy; Abbvie: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees. Patel:Exelixis: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.