Abstract
Background: Cytopenias are associated with worse survival and symptoms in patients with MF. Patients with thrombocytopenia (platelet counts <100 x109/L) have worse quality of life and higher symptom burden based on the MPN Symptom Assessment Form Total Symptom Score (TSS) instrument, particularly in the areas of fatigue, inactivity, and early satiety. However, thrombocytopenia and anemia frequently co-occur, and it is unknown to what extent the association between thrombocytopenia and symptom burden is driven by anemia. In this analysis of TSS data from two randomized trials, we sought to understand the differential impact of thrombocytopenia and anemia on symptom burden.
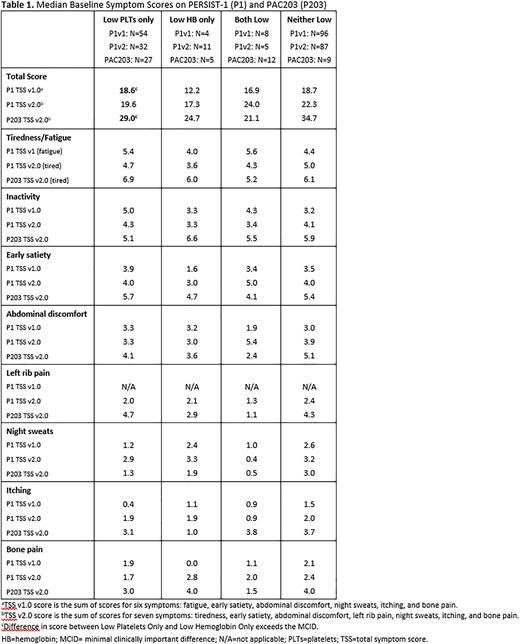
Methods: Data from PERSIST-1 (Phase 3; NCT01773187) and PAC203 (Phase 2; NCT04884191) were included, as both studies enrolled patients irrespective of baseline hemoglobin (HB) or platelet (PLT) count. Patients were included if they had not received prior ruxolitinib within 30 days prior to randomization. The TSS version completed by patients was v1.0 for the first 55% of patients enrolling on PERSIST-1 and v2.0 for the remainder of PERSIST-1 and all PAC203. All symptoms in TSS v2.0 were assessed, as were symptoms that were common between v1.0 and v2.0. Symptoms scores were compared between patients with low platelets only (PLT count <100x109/L and HB ≥8 g/dL) and low HB only (PLT count ≥100x109/L and HB <8 g/dL). In addition, patients with both and neither counts low were analyzed. A difference in total score of 4.3 was considered the minimal clinically important difference (MCID, Mesa RA et al, J Clin Oncol. 2013;31(10):1285-92).
Results: Among the 351 evaluable patients, 113 and 20 were in the low PLTs only and low HB only groups, respectively. Additionally, 25 had both low PLTs and HB, while 193 had neither low. The patients in both the low PLT and the low HB groups were divided approximately 3:1 between PERSIST-1 and PAC203. By contrast, the group with both counts low was divided approximately 1:1 between the two studies, whereas the group with neither count low was derived almost entirely from PERSIST-1 (95%) rather than from PAC203 (5%).
Patients with low PLTs (vs low HB) were farther from initial MF diagnosis (3.2 vs 0.7 years), were more likely to have ECOG performance status ≥2 (23% vs 15%), and had larger median spleen volumes (2475 vs 2193 cm3). There was no difference in age (69 vs 69 years), proportion with primary MF (74% vs 75%), proportion with prior JAK inhibitor exposure (24% vs 25%), or median white blood cell count (9.8 vs 7.6 x109/L).
Patients with low PLTs had consistently higher TSS scores, indicating more severe symptoms, compared to patients with low HB (Table 1). The difference in scores between the low PLT and low HB groups exceeded the MCID on PERSIST-1 TSS v1.0 (median 18.6 vs 12.2) and PAC203 (median 29.0 vs. 24.7). The higher total scores seen with low PLTs were driven by higher symptom burden in tiredness / fatigue, as well as in spleen-related symptoms (early satiety, abdominal discomfort, and left rib pain). Smaller differences were observed among the cytokine-related symptoms (night sweats, itching, and bone pain), with higher scores observed in the low HB group.
Discussion: These data suggest that anemia may not be the sole driver of symptom burden among patients with cytopenic MF. Surprisingly, patients with isolated thrombocytopenia appear to have more profound symptom burden than those with isolated anemia. While anemia is often associated with reduced physical function and increased fatigue, our data show that isolated thrombocytopenia has a profound impact on tiredness and fatigue in the setting of MF. The mechanism underlying the association between thrombocytopenia and MF symptoms is not known, though thrombocytopenia is a strong marker of advanced disease. While this analysis is limited by smaller sample size for some groups, these data highlight the specific symptom profile of patients with MF who have isolated thrombocytopenia, as compared to patients with isolated anemia. In turn, patients with MF and thrombocytopenia may benefit from symptom- and disease-targeting therapy.
Disclosures
Palmer:Protagonist: Consultancy; Sierra Oncology: Consultancy; CTI BioPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Research Funding. Gerds:Incyte Corporation: Research Funding; Accurate Pharmaceuticals: Research Funding; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago BioSciences: Research Funding; Kratos Pharmaceuticals: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Harrison:EHA: Other: Leadership role; Incyte: Speakers Bureau; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Promedior: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sierra: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; MPN voice: Other: Leadership role; Gilead: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Galacteo: Membership on an entity's Board of Directors or advisory committees. Kiladjian:AOP Orphan: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Buckley:CTI BioPharma: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Roman-Torres:CTI BioPharma: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Mascarenhas:Forbius: Research Funding; Kartos: Consultancy, Research Funding; Sierra Oncology: Consultancy; Prelude Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy; Merus: Research Funding; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Galecto: Consultancy; PharmaEssentia: Consultancy, Research Funding; Merck: Research Funding; AbbVie: Consultancy, Research Funding; Janseen: Research Funding; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago: Consultancy; Roche: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Mesa:CTI: Research Funding; Geron: Consultancy; AOP: Consultancy; Sierra Oncology: Consultancy, Research Funding; Novartis: Consultancy; Samus: Consultancy, Research Funding; LaJolla Pharmaceutical: Consultancy; Celgene: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Genotech: Research Funding; Promedior: Research Funding; Roche: Consultancy; Blueprint: Consultancy; Bristol Myers Squibb: Consultancy; AbbVie: Research Funding; Gilead: Research Funding; Incyte: Consultancy, Research Funding; Imago: Research Funding.
OffLabel Disclosure:
Pacritinib is a kinase inhibitor indicated for the treatment of adults with intermediate or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis with a platelet count below 50 Ã- 10^9/L. This indication is approved under accelerated approval based on spleen volume reduction. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Author notes
Asterisk with author names denotes non-ASH members.