Abstract
Background: Most acute lymphoblastic leukemia (ALL) patients are treated with chemotherapy as primary care. Although treatment response is usually positive, resistance and relapse often occur through unclear mechanisms. Ferroptosis is an iron-dependent form of regulating cell death associated with cancer. The characteristics of ferroptosis in ALL are still uncertain. This study aimed to explore the effect of ferroptosis on drug resistance and prognosis, and the potential of ferroptosis-based treatment in ALL.
Methods: We comprehensively evaluated the ferroptosis levels of 480 ALLs from the TARGET and GEO database, and 99 ALLs from our clinic based on 487 ferroptosis-related specific genes (FRGs) downloaded from FerrDb database. The ferroptosis score (FS) was constructed to quantify the ferroptosis levels of individuals using principal component analysis algorithms. Next, the prognostic value and therapeutic sensitivities were evaluated using multiple methods. We then assessed the transcription profile of 99 ALLs in our clinic according to the minimal residual diseased (MRD) level, and the integrated metabolic analysis and RNA sequencing in drug-resistant and sensitive ALL cell lines. The biological function of solute carrier family 7 member 11 (SLC7A11), one of FRGs, on cell growth, lipid peroxidation, and drug sensitivity were further examined in ALL cell lines Nalm-6, Jurkat, and their corresponding drug-resistant cells Nalm-6/ADR and Jurkat/ADR.
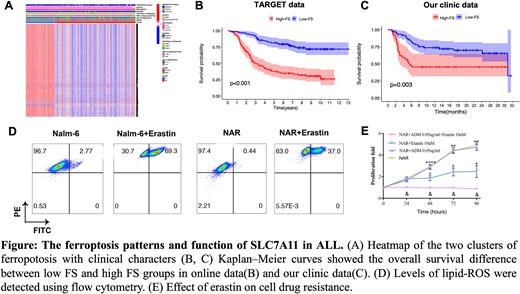
Results: At first, we identified that FRGs have prevalent genetic alteration in ALL, most for missense mutation of driver genes. In addition, the heterogeneous expression of the FRGs in normal and ALL samples indicated the involvement of FRGs expression in ALL development. Subsequently, we identified two distinct ferroptosis phenotypes in ALL with significantly different prognoses. We then developed a risk model termed FS to evaluate ferroptosis levels of ALL patients based on differential expression genes between the two ferroptosis phenotypes. Compared with the low-FS group, the high-FS group was characterized by higher estimated IC50 in the many chemotherapy drugs with an unfavorable prognosis, which was validated by our clinical data. Furtherly, SLC7A11 was identified as a key regulator of ferroptosis-related drug resistance in ALL. Clinically, SLC7A11 was selected out with the highest log |FC| in the MRDHigh(>1%) group in comparison with MRDlow(<0.01%) group. The abundance of SLC7A11 was strongly correlated with relapsed/refractory disease. Overexpression of SLC7A11 and reduction of ferroptosis were observed in the drug-resistant cells. The SLC7A11-specific classic ferroptosis inducer erastin and shRNA both decreased cellular glutamine uptake, increased cellular lipid peroxidation, enhanced the sensitivity of chemotherapeutic with synergism, and led to deceleration of cell proliferation via glutamate metabolism in the oxidative phosphorylation pathway.
Conclusions: Ferroptosis score could be a prognostic indicator for ALL progression and chemotherapy resistance. SLC7A11 functioned as an extracellular glutamine transporter, promoting ALL resistance through reprogramming glutamine metabolism of ALL, while rendering ALL cells insensitive to chemotherapy. Ferroptosis-based therapies employing agents to target SLC7A11 offered substantial therapeutic improvements over conventional chemotherapy for ALL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.