Abstract
Background: Ide-cel received regulatory approval for the treatment of patients with RRMM after ≥4 prior lines of therapy based on the results of the pivotal KarMMa trial in which patients achieved an overall response rate (ORR) of 73%, ≥ complete response (CR) in 33%, and median progression free survival (PFS) of 8.8 months (Munshi et al. N Engl J Med 2021). Patients who had previously received a BCMA-targeted therapy (BCMA-TT) were excluded from the KarMMa trial. We evaluated the real-world outcomes for patients treated with standard of care ide-cel after having previously received a BCMA-TT.
Methods: Eleven US academic centers contributed data to this effort which included patients who had undergone apheresis up until 5/1/2022, and who were infused with ide-cel with sufficient follow-up duration for at least a day 30 response assessment. Patients who died from an infection or ide-cel related toxicity prior to response assessment were included in the safety and survival analyses, but were not considered evaluable for response.
Results: A total of 50 patients who had received a prior BCMA-TT and were later infused with ide-cel were evaluable for safety and survival analyses, of which 49 were evaluable for response. The specific type of prior BCMA-TT with respective ORR to the prior BCMA-TT were antibody-drug conjugates (n=38, ORR 17%), bispecifics (n=7, ORR 0%), and CAR T (n=5, ORR 80%). This cohort consisted of 56% patients aged ≥ 65 years, 66% male, and 19% with ECOG performance status ≥ 2. Disease characteristics were notable for 36% with high-risk FISH findings as defined by del(17p), t(4;14), and t(14;16), 50% extramedullary disease, 27% R-ISS stage III disease, and 30% with high bone marrow plasma cell burden (≥50%) prior to ide-cel infusion. Patients were heavily pre-treated with a median of 9 prior lines of therapy, 88% received prior autologous stem cell transplant, 62% were penta-refractory, and 86% required bridging therapy (68% with ≥SD on bridging therapy). Compared to the cohort of patients who had not received a prior BCMA-TT (n=153), patients who received prior BCMA-TT were more likely to have t(4;14) as a high-risk feature (23% v 8%; p=0.005), had a higher median number of prior lines of therapy (9 v 6; p<0.0001), and were more likely to have penta-refractory disease (62% v 37%; p=0.002).
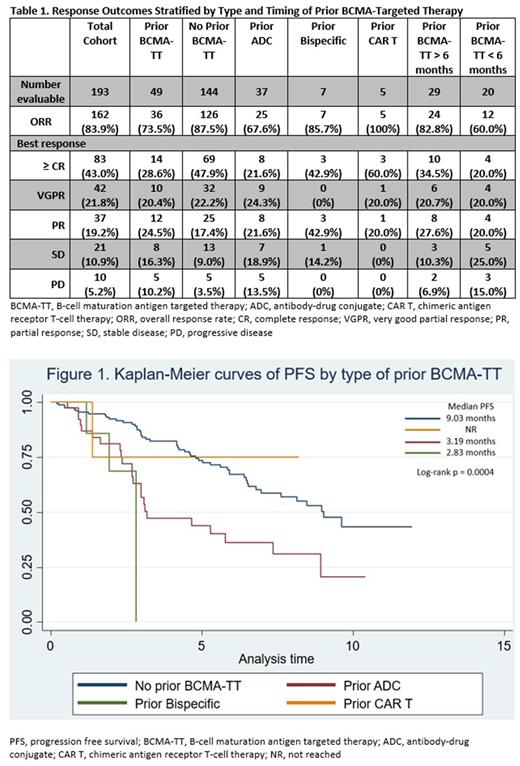
Response outcomes stratified by the specific type of prior BCMA-TT and by timing of prior BCMA-TT are summarized in Table 1. Patients receiving prior BCMA-TT had a lower ORR (74% v 88%; p=0.021) and lower best response of ≥ CR (29% v 48%; p=0.018) compared to those without prior BCMA-TT. Compared to patients who were treated with a BCMA-TT > 6 months prior to ide-cel, those with treatment < 6 months prior to ide-cel had lower rates of overall response (60% v 83%; p=0.076), CR (20% v 34.5%; p=0.22), and median PFS (3.0 v 5.3 months; p=0.39), though these differences did not reach statistical significance. Treatment with a prior BCMA-TT was associated with an inferior median PFS compared to those without prior BCMA-TT (3.2 v 9.0 months; log-rank p=0.0002). The 3-month PFS rates amongst those with prior ADC, prior bispecific, prior CAR T, and without prior BCMA-TT were 57.2%, 41.5%, 77.8%, and 85% respectively. On univariate analysis amongst patients treated with a prior BCMA-TT, having penta-refractory disease reached borderline significance for association with response of < PR (p=0.053), and treatment with a prior ADC trended for association with a response of < CR compared to other modalities of BCMA-TT (p=0.076).
The toxicity profile for patients receiving a prior BCMA-TT was similar to those in our cohort who had not received a prior BCMA-TT and to that described in KarMMa. Grade ≥ 3 CRS and ICANS were 2.0% and 8.5% respectively. Patients in the prior BCMA-TT cohort were more likely to have grade 4 thrombocytopenia in the first 30 days after ide-cel infusion (46% v 31.5%; p=0.064) and were more likely to receive a thrombopoietin (TPO) agonist (27% v 12%; p=0.017).
Conclusions: This multicenter retrospective study characterizes a large cohort of patients who received a prior BCMA-TT before treatment with ide-cel, which is associated with inferior PFS and less likelihood of attaining both an overall response and best response of ≥CR. There was a trend towards worse efficacy outcomes for patients who received ide-cel < 6 months after their prior BCMA-TT, and the timing of ide-cel infusion after prior BCMA-TT warrants further investigation.
Disclosures
Ferreri:Affimed: Current equity holder in publicly-traded company; Sanofi: Membership on an entity's Board of Directors or advisory committees. Hashmi:Sanofi: Consultancy, Speakers Bureau; GSK: Speakers Bureau; KARYOPHARM: Speakers Bureau; JANSSEN: Consultancy; BMS: Consultancy. McGuirk:Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Orca Bio: Research Funding; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial; Nextar: Consultancy, Honoraria; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau. Sborov:Sanofi: Consultancy; BMS: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bioline: Consultancy; Amgen: Consultancy; Pfizer: Consultancy. Wagner:Abbvie Inc.: Other: Partner is currently employed as a Medical Science Liaison . Atrash:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Takeda: Honoraria; Celgene: Honoraria, Speakers Bureau; GSK: Honoraria, Research Funding; Amgen: Research Funding. Voorhees:Janssen: Consultancy; Karyopharm Therapeutics: Consultancy; Oncopeptides: Consultancy; AbbVie: Consultancy; Bristol Myers Squibb: Consultancy, Other: Data Safety and Monitoring; Sanofi: Consultancy; GSK: Consultancy; Pfizer: Consultancy. Simmons:Kite/Gilead: Speakers Bureau. Alsina:BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Research Funding. Locke:BioPharm Communications: Other: Education or editorial activity; ASH: Other: Education or editorial activity; Leukemia and Lymphoma Society: Research Funding; Aptitude Health: Other: Education or editorial activity; ), National Cancer Institute: Research Funding; CERo Therapeutics: Research Funding; Takeda: Consultancy; Sana: Consultancy; BMS: Research Funding; Daiichi Sankyo: Consultancy; CAREducation: Other: Education or editorial activity; Clinical Care Options Oncology: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; Society for Immunotherapy of Cancer: Other: Education or editorial activity; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy. Sidana:Prothena: Honoraria; Oncopeptides: Consultancy; Sanofi: Consultancy; Janssen: Consultancy, Research Funding; Magenta Therapeutics: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Allogene: Research Funding. Hansen:BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria; Survivorship: Honoraria. Patel:Janssen, Celgene/BMS, Caribou Sciences, Arcellx, Cellectis, Merck, Pfizer, Karyopharm, Oncopeptides: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.