Abstract
Introduction: Despite improvements in the treatment of mantle cell lymphoma (MCL) over the last 10-15 years, MCL remains incurable with the majority of patients experiencing multiple relapses. BTK inhibitors (BTKi) are active in R/R MCL, but duration of response is typically short, and there are only few treatment options in the post BTKi setting. Other active agents in R/R MCL are venetoclax, and the combination of lenalidomide and rituximab (R2). We evaluated safety and efficacy of this triplet in the multi-centre, open-label, phase Ib-II trial in R/R MCL in BTKi failed and in BTKi-naïve patients. Another objective of this trial was to test the feasibility of stopping treatment in patients achieving molecular remission.
Methods: Main eligibility criteria were a diagnosis of MCL, progressing after or being refractory to at least one rituximab-containing chemotherapy regimen, WHO PS 0-3, and measurable disease. Primary endpoint was overall response rate (ORR) at 6 months. Minimal residual disease (MRD) was monitored by RQ-PCR every 3 months during follow-up, according to EuroMRD criteria. Sequencing of the most frequently mutated genes in MCL was performed on frozen tumor cells at the time of relapse, as well as assessment of ctDNA in plasma at baseline and during treatment.
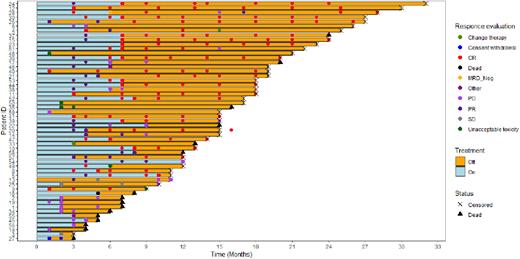
Results: In total, 59 patients (pts) with a median age of 73 years were enrolled. 48 (81%) were males and 22 (37%) were MIPI high risk. Median number of previous treatment lines was 2 (range x-x); 15 (25%) had progressed after a BTKi, and 26 (44%) had previously received autologous stem cell transplant. At 6 months, ORR was 63% (29 CR, 8 PR). Among the 15 pts previously exposed to BTKi, 6 (40%) responded at 6 months (4 CR, 2 PR). 20 pts stopped treatment early due to PD (n=17; 29%) or AE (n=3; 5%). At 24 months, 49% of the pts were free from progression, and 59% were alive. 6 pts (10%) did not have MCL involvement in the BM and were followed with PET-CT. 35 pts (59%) stopped treatment in CR and molecular remission. Molecular relapse was detected in 4/35 (11%) pts, who restarted treatment after 12 to 14 months.
Hematological toxicity was the most frequent AE; 52/59 (88%) pts with G3-4 neutropenia, requiring G-CSF support, and 21/59 (36%) pts with G3-4 thrombocytopenia. 8 events of G3-4 infection were recorded (14%). Rash occurred in 21 pts (36%), of which 5 were G3. One patient died from PML after 2 months of treatment. Tumor lysis syndrome was not observed.
Conclusions: Combination of venetoclax of 600 mg and lenalidomide 15 mg is tolerable and efficacious in pts with R/R MCL. Neutropenia was frequent and required G-CSF support. An MRD response-adapted treatment strategy with treatment discontinuation in MRD negative pts. and reinitiating upon MRD positivity was feasible. Updated results will be presented at the meeting, including outcome in relation to histology, TP53 mutational status and ctDNA analysis.
Disclosures
Jerkeman:AstraZeneca: Honoraria, Research Funding; Genmab: Honoraria; Kite/Gilead: Consultancy, Honoraria, Research Funding; Incyte: Honoraria; Orion: Honoraria; Novartis: Honoraria; Roche: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding. Hutchings:AbbVie: Consultancy; Celgene: Consultancy, Research Funding; Celgene, Genentech, Genmab, Incyte, Janssen, Novartis, Roche, Takeda: Research Funding; Genmab: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Genentech: Research Funding; Roche: Consultancy, Research Funding; Incyte: Research Funding; Novartis: Research Funding; Takeda: Consultancy, Research Funding; AbbVie, Celgene, Genmab, Janssen, Roche, Takeda: Membership on an entity's Board of Directors or advisory committees. Niemann:Genmab: Consultancy; Abbvie: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Janssen: Consultancy; Octapharma: Consultancy, Research Funding; CSL Behring: Consultancy; Takeda: Consultancy; Beigene: Consultancy. El-Galaly:Abbvie: Other: Teaching in 2021; Roche: Ended employment in the past 24 months. Riise:AstraZeneca: Consultancy. Glimelius:Takeda: Research Funding; Janseen Cilag: Research Funding.
OffLabel Disclosure:
Venetoclax for relapsed/refractory MCL
Author notes
Asterisk with author names denotes non-ASH members.