Abstract
Background The Canadian Registry for Amyloidosis Research (CRAR) is a nationwide disease registry with a targeted dataset focused on transthyretin (ATTR) and light-chain (AL) amyloidosis, which currently includes eight academic referral centres across Canada.
Objectives: We describe the development of a linked clinic-reported and patient-reported registry dataset for evidence to characterize patient profiles, care environments and clinical outcomes for amyloidosis patients in Canada, and to understand the use, safety, and effectiveness of amyloidosis therapies in real-world settings.
Methods: CRAR established a registry governance to oversee operation of a robust amyloidosis registry with broad input from multi-disciplinary perspectives. The steering committee is made up of clinical experts from cardiology, hematology, and neurology, as well as patient organization representatives. A multi-stakeholder consensus process involving clinical experts from multiple medical subspecialties, patient organization representatives, and industry partners was undertaken to derive a common core dataset aligned with varied perspectives, and to ensure utility of a well-rounded program. The dataset consensus process included multiple surveys to rank data items for importance, culminating in a consensus in-person meeting with the steering committee. Alongside consensus building for a clinic-reported dataset, patients and patient organizations were engaged in development and implementation of a patient-reported dataset.
Results: Consensus registry objectives were defined as:
To assemble a comprehensive dataset describing real-world care of amyloidosis patients in the Canadian Context.
To define the impact of ATTR and AL treatments on clinical symptomology, comorbidities, and mortality.
To advance the understanding of treatment outcomes to improve/enable the long-term follow-up of patients.
To empower the Canadian community and provide a platform for collaborative research studies.
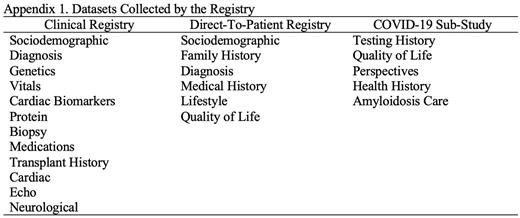
The consensus registry dataset includes items that address access to care and outcomes for Canadians with amyloidosis, therapy effectiveness and safety, and is collected in a multi-centre, multi-disciplinary, prospective, observational study of amyloidosis patients regardless of therapeutic status. Data items collected include: sociodemographics, diagnosis, genetics, cardiac biomarkers, protein studies, biopsy, medications, transplant history, cardiac results, and neurological results. Additionally, the consensus dataset includes the patient perspective including quality of life and activities of daily living. Both prospective and retrospective (including deceased) patient cohorts will be included.
Further baseline data will be presented on an initial cohort of patients.
Conclusion CRAR has been established to collect a prospective and retrospective, longitudinal, multidisciplinary dataset that will help evaluate ATTR and AL amyloidosis care and outcomes.
CRAR has successfully launched at multiple sites and is expanding to additional specialty amyloidosis centres across Canada. The growth of this program will promote opportunities to perform multi-institutional observational studies that provide real-world efficacy and safety data and inform cost-effectiveness of therapies, while supporting patient recruitment for clinical trials and engaging patients in the collection of outcome data (Davis, M. & Fine, N., 2020).
The Canadian Registry for Amyloidosis Research has obtained operational funding support from Pfizer, Akcea, Eidos Therapeutics, Servier, Boehringer-Ingelheim, and Takeda. These funders have not been involved in the planning, development, or analysis of any CRAR activities.
Appendix Appendix 1. Datasets Collected by the Registry
References Davis MK, Fine NM. An Urgent Need for Data to Drive Decision Making: Rationale for the Canadian Registry for Amyloidosis Research. Can J Cardiol. 2020 Mar;36(3):447-449. doi: 10.1016/j.cjca.2019.12.005. Epub 2019 Dec 10. PMID: 32044152.
Disclosures
Venner:FORUS Therapeutics: Honoraria; Pfizer: Honoraria; Janssen: Honoraria; Takeda: Honoraria; GSK: Honoraria; Sanofi: Honoraria; BMS: Honoraria. Reece:Amgen: Consultancy, Honoraria; BMS: Research Funding; Merck: Research Funding; Karyopharm: Consultancy; Otsuka: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria; Millenium: Research Funding; GSK: Honoraria. Jimenez-Zepeda:Janssen: Honoraria; Amgen: Honoraria; BMS: Honoraria; GSK: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; FORUS Therapeutics: Honoraria; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.