Abstract
Background:
Poor graft function (PGF) is a known life-threatening complication following the allogeneic stem cell transplantation (Allo-SCT) characterized by multilineage cytopenia in the absence of mixed donor chimerism (<95% donor), relapse, or graft-versus-host disease (GVHD). The incidence of PGF after Allo-SCT is 5-20%, with limited treatment options. We present a systematic review and meta-analysis to assess the efficacy of thrombopoietin-mimetic agent Eltrombopag (EPAG) for PGF in adult Allo-SCT recipients.
Method:
A literature search was performed on four databases (PubMed, Cochrane Register of Controlled Trials, Embase and Clinicaltrials.gov) using MeSH terms and keywords for "Leukemia, Plasma Cell" AND "Hematopoietic Stem Cell Transplantation" AND "Receptors, Thrombopoietin" OR "Eltrombopag" from the date of inception to March 09, 2022. We selected seven retrospectives studies after screening 1034 articles, reporting data on EPAG efficacy for PGF following Allo-SCT in adult patients. Data were extracted following the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines. Quality evaluation was performed using the NIH quality assessment tool. The inter-study variance was calculated using Der Simonian-Laird Estimator. The pooled analysis was conducted using the 'meta' package by Schwarzer et al. in the R programming language (version 4.16-2). A random-effects model was used to estimate the proportions with 95% confidence intervals (CI).
Results:
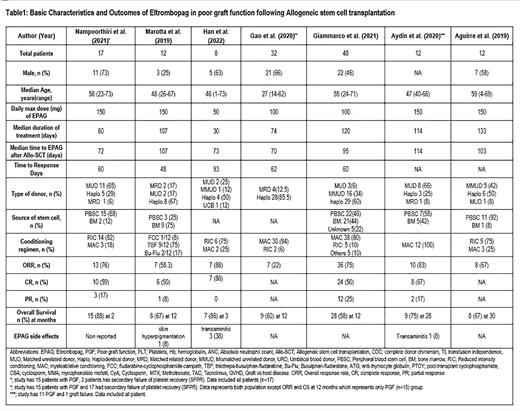
A total of 141 patients from seven studies were included in this systematic review and meta-analysis. (Table 1) Six studies reported that the median age of patients was 48.5 (18-73) years, and 53.4% were males. The median daily dose of EPAG was 150 (50-150) mg, while the median duration of treatment was 114 (30-133) days. The median time to EPAG administration after Allo-SCT was 95 (70-114) days and the median time to response was 60 (48-93) days, as reported by five studies. The source of stem cells was peripheral blood stem cells and bone marrow in 57.42% (58/101) and 37.62% (38/101) patients, respectively, as reported by five studies. The donor type was haploidentical 59% (83/141), matched unrelated donor (MUD) 19% (27/141), mismatched unrelated donor (MMUD) 15.6% (22/141), matched related donor (MRD) 5.7% (8/141), and umbilical cord blood (UCB) 0.7% (1/12). The pooled overall response rate (ORR) was 66% (95% CI 0.45-0.84, p<0.01, I 2 = 81%) while pooled complete response (CR) and pooled partial response (PR) was 58% (95 % CI 0.46-0.69, p= 0.33, I 2 =14%) and 19.5% (95% CI 0.11-0.29, p= 0.68, I 2 = 0%), respectively. At a median follow-up of 12 (2-30) months, the median pooled overall survival (OS) was 66% (95 % CI 0.47-0.82, p< 0.01, I 2 =75%). One study reported skin hyperpigmentation as a side effect of EPAG in 1/12 (8.3%) patients, and two studies reported transaminitis as a side effect in 4/20 (20%) patients.
Conclusion:
Eltrombopag could provide an effective treatment option for poor graft function after Allo-SCT with an acceptable toxicity profile. However, there is an unmet need for randomized trials to address the optimal dosing and duration of Eltrombopag treatment for PGF after Allo-SCT.
Disclosures
Mahmoudjafari:Omeros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees; Merk: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Abhyankar:Incyte: Consultancy, Research Funding, Speakers Bureau; Therakos: Consultancy, Research Funding, Speakers Bureau. McGuirk:Magenta Therapeutics: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Nextar: Consultancy, Honoraria; Orca Bio: Research Funding; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial.
Author notes
Asterisk with author names denotes non-ASH members.