Abstract
Heparan sulfate proteoglycans (HSPGs) are expressed at the cell surface and in the extracellular matrix, including within the bone marrow microenvironment. Heparan sulfate (HS) is the glycosaminoglycan side chain of HSPGs, and these side chains bind to a myriad of growth factors, cytokines, chemokines, and extracellular matrix molecules, mediating a diverse range of biological functions. It is well established that HS-protein interactions critically depend on the amount and position of 6-O-sulfate groups, which form binding sites for proteins. The HS 6-O-endosulfatases, the Sulfs (Sulf1 and Sulf2), remove 6-O-sulfates from specific HS intra-chain sites and are the only known enzymes that can modify HS sulfation status extracellularly. A gain or a loss of HS 6-O-sulfation can alter the outcome of HS-ligand interactions, such as with Wnt ligands, fibroblast growth factors (FGF), and transforming growth factor (TGF)-β1. Monocytes and macrophages express the Sulfs, predominantly Sulf2, and their secretion of the Sulfs can influence HS sulfation status in their surrounding microenvironments. The objective of this study was to examine the impact of myeloid lineage Sulf deletion on hematopoiesis.
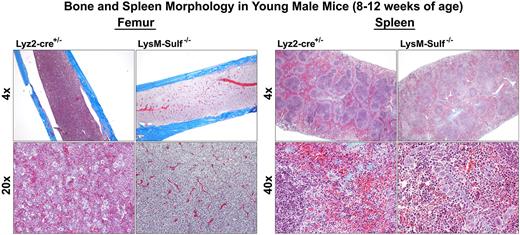
For this investigation, we generated a murine model of Sulf deletion in Lysozyme 2 (Lyz2) positive myeloid lineage cells by crossing Sulf1 and Sulf2 double floxed mice with the LysMcre mouse line (hereinafter LysM-Sulf-/-). At 8-12 weeks of age, the bone marrow of LysM-Sulf-/- male mice displayed myelodysplastic changes with reduced number of megakaryocytes (MK) compared to the control mice (Lyz2-cre+/-). Bone marrow-derived (BMD) macrophages from LysM-Sulf-/- mice exhibited enhanced proliferation but reduced phagocytosis of Escherichia Coli (E. Coli)in vitro. In addition, LysM-Sulf-/- BMD macrophages expressed reduced levels of key inflammatory mediators including inducible nitric oxide synthase (iNOS), tumor necrosis factor (TNF)-α and interferon gamma-induced protein 10 (IP-10) in response to lipopolysaccharide stimulation compared to control BMD macrophages. Complete blood cell counts revealed decreases in total white blood cells (4.6 ± 0.6 10E9 Cells/L vs. 8.1 ± 1.010E9Cells/L in control mice, P = 0.0193), red blood cells (RBC, 10.9 ± 0.1 10E12 Cells/L vs. 11.4 ± 0.2 10E12 Cells/L in control mice, P = 0.0385), and hematocrit (43.5 ± 0.3% vs. 46.3 ± 0.7% in control mice, P = 0.0054) in LysM-Sulf-/- males. In addition, LysM-Sulf-/- males (8-12 weeks of age) exhibit extramedullary hematopoiesis with splenomegaly (spleen size increased by 2.6-fold, 162.1 ± 11.5 mg vs. 79.1 ± 1.2 mg in control mice, P < 0.0001). Bone and spleen morphologies are shown in the accompanied Figure. In older LysM-Sulf-/- males (8-12 months of age), the reductions in RBC and hematocrits were further exacerbated with increased reticulocyte counts in the peripheral blood. In addition, thrombocytopenia was observed in 8-12 months old LysM-Sulf-/- males (platelet counts 125.8 ± 41.4 vs. 483.3 ± 7.1 in control mice, P = 0.0007). The above changes were accompanied by amplified splenomegaly (spleen size increased by 9.2-fold, 860.4 ± 157.6 mg vs. 93.2 ± 8.2 mg in control mice, P = 0.0152) and erythropoiesis in the liver. In terms of signaling, enhanced Smad2/3 phosphorylation (primary transducers of TGF-β1 signaling) was observed in both the spleens and the lungs of LysM-Sulf-/- male mice. Finally, in contrast to the male mice, complete blood cell counts were normal in both young and old LysM-Sulf-/- females without obvious extramedullary hematopoiesis.
Our investigation revealed the importance of HS 6-O-sulfation in supporting normal hematopoiesis in the bone marrow, and alterations in HS 6-O-sulfation status in the bone marrow microenvironment due to LysM-Sulfdeletion leads to not only impairment in monocyte/macrophage development and function, but also age- and sex-dependent deficiencies in erythropoiesis and thrombopoiesis. Our study identifies TGF-β1 as one of the signaling pathways regulated by the Sulfs. The regulatory roles of the Sulfs in TGF-β1 as well as other signaling pathways in hematopoiesis warrant further investigation.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.