Abstract
Background: Multiple myeloma (MM) is an incurable malignancy associated with significant morbidity. The recent expansion of available treatment choices has increasingly complicated later-line treatment decision-making, where standard of care (SOC) is not necessarily established. However, the patient (pt) and hematologist/oncologist (HEM/ONC) priorities that underlie treatment decision-making in real-world settings for MM have not been well studied. We aimed to better understand these factors using qualitative methods.
Methods: In March 2021, we conducted in-depth interviews with 12 adults with MM (11 from the US and one from Canada) and 12 HEM/ONCs in the US (seven from community settings, five from academic settings; all of whom spend ≥50% of their time in direct pt care and currently manage ≥10 pts with active MM). A commercial healthcare database was used to recruit HEM/ONCs, and a pt advocacy group, the HealthTree Foundation for Multiple Myeloma, was used to recruit pts. A trained moderator used a semi-structured interview guide to facilitate discussion of MM treatment decision-making. NVivo, a qualitative analysis software program, identified key themes emerging from the interviews.
Results: HEM/ONCs had a median of 17 years (range 5-30) in practice. Pts had a median age of 64 years (range 49-70) and a median time since MM diagnosis of 6 years (range 1-8).
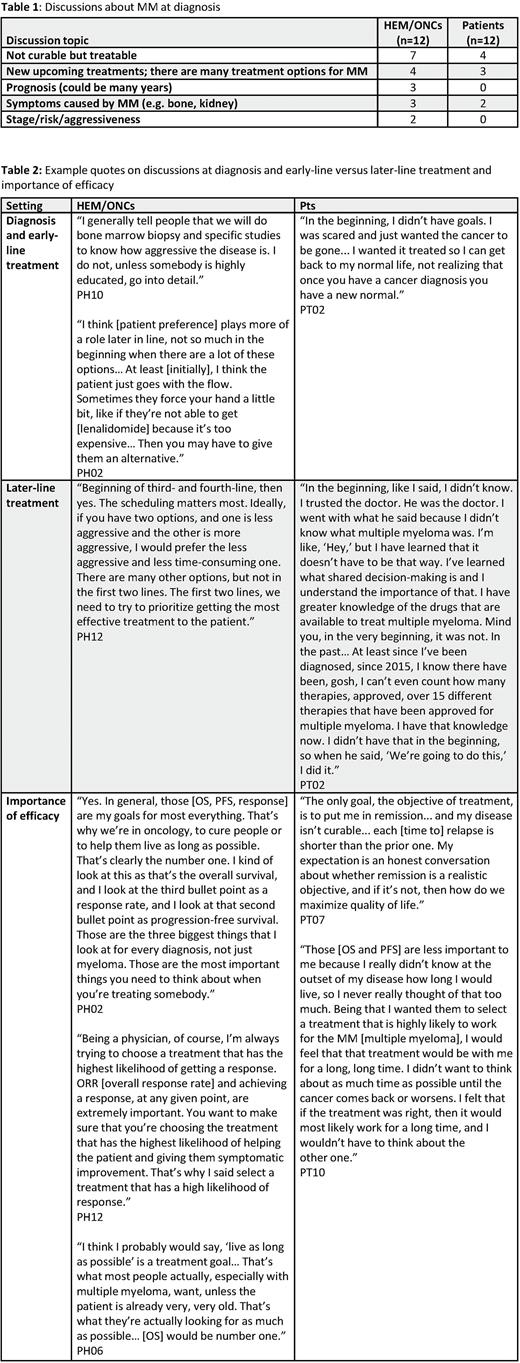
At the time of MM diagnosis, HEM/ONCs described MM as treatable with many therapeutic options. They noted that discussions of risk status, cytogenetics, and biomarkers can be difficult for pts to understand; as these factors may not always influence early-line treatment decisions, HEM/ONCs reported simplifying or delaying such discussions, instead focusing on immediate next steps such as the treatment plan. HEM/ONCs also recommended first-line SOC comprising lenalidomide/bortezomib/dexamethasone (n=9/12), regardless of pt risk status.
Similarly, pts reported that initial MM diagnosis discussions focused on the treatment they would receive (n=8/12). Discussions with their treating physician were focused predominantly on treatability (n=4/12), reassurance (n=3/12), and symptom control (n=2/12), rather than individualized factors or therapy. No pts recalled detailed discussions of risks or cytogenetics at initial diagnosis (n=0/12) (Table 1).
In treatment discussions for later lines of therapy, HEM/ONCs appear to offer more options and pts appear to participate more in treatment decisions: pts reported their preferences were considered for later-line therapy (n=5/12), for switching treatments due to side effects (n=3/12), and when offered a 'brand new' medication (n=2/12); HEM/ONCs also reported shared decision-making for later-line therapy (n=7/12; Table 2).
Regarding treatment goals, all MM pts (regardless of line of therapy; n=12/12) and HEM/ONCs (n=12/12) prioritized efficacy. Among efficacy measures, pts prioritized response (n=10/12) and overall survival (OS; n=7/12), followed by progression-free survival (PFS; n=4/12). These efficacy goals aligned with the top treatment goals for HEM/ONCs (response: n=12/12; OS: n=11/12; PFS: n=8/12). Aside from efficacy, priorities for pts and HEM/ONCs overlapped on ability to do physical activities (n=4/12 pts, n=6/12 HEM/ONCs), participating in social activities (n=3/12 pts, n=6/12 HEM/ONCs), getting back to normal life (n=3/12 pts, n=4/12 HEM/ONCs), continuing to work (n=3/12 pts, n=4/12 HEM/ONCs), and maintaining a family role (n=2/12 pts, n=3/12 HEM/ONCs). Areas prioritized by pts but not by HEM/ONCs included avoiding being a burden on their family (n=5/12 pts, n=0/12 HEM/ONCs), and avoiding serious heart damage (n=5/12 pts, n=1/12 HEM/ONCs) and kidney damage (n=4/12 pts, n=1/12 HEM/ONCs). HEM/ONCs prioritized avoiding neuropathy and convenience of administration, which pts did not mention.
Conclusions: The pt's role in treatment selection increases in later lines as they are offered more options, receive more information, and feel their preferences are considered. In the current study, both pts and HEM/ONCs prioritized disease control over most other aspects, particularly in later lines. Pts and HEM/ONCs prioritized maintaining quality of life over avoiding specific adverse events that are not life-threatening or requiring medical intervention.
Disclosures
Shah:TeneoBio: Research Funding; Sutro Biopharma: Research Funding; GSK: Consultancy; CSL Behring: Consultancy; Sanofi: Consultancy; Poseida: Research Funding; Nektar: Research Funding; Karyopharm: Consultancy; Janssen: Research Funding; CareDx: Consultancy; BMS/Celgene: Consultancy, Research Funding; Amgen: Consultancy; AstraZeneca: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Oncopeptides: Consultancy; Precision Biosciences: Research Funding; Indapta Therapeutics: Consultancy; Bluebird Bio: Research Funding; Allogene: Consultancy; Kite: Consultancy. Mearns:Genentech: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Carey:Kantar: Ended employment in the past 24 months; Cerner Enviza: Current Employment. Mulvihill:AstraZeneca: Consultancy, Research Funding; Cerner Enviza: Current Employment. McClerklin:National Science Foundation (ended 2021): Research Funding; University of Chicago: Ended employment in the past 24 months; Cerner Enviza: Current Employment. Thomson:Cerner Enviza: Current Employment. Ahlstrom:Pfizer, BMS, Janssen, Takeda Oncology, Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Girvan:Eli Lilly: Current equity holder in publicly-traded company, Ended employment in the past 24 months; AbbVie: Current Employment, Current equity holder in publicly-traded company. Cornell:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Nixon:Roche / Genentech: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.