Abstract
Background
Rusfertide (PTG-300) is a hepcidin mimetic that binds to ferroportin, decreasing availability of iron in bone marrow and reducing erythrocytosis. Accordingly, rusfertide is in development for treatment of conditions characterized by excessive erythropoiesis, such as polycythemia vera (PV). In the phase 2 REVIVE study (NCT04057040), patients with therapeutic phlebotomy (TP)-dependent (i.e., ≥3 phlebotomies within a 6-month period) PV were treated with rusfertide with or without cytoreductive therapy. Rusfertide resulted in sustained control of hematocrit (HCT) at <45% and eliminated the requirement for TP in 84% of patients [Hoffman, ASH 2021].
Methods
REVIVE is comprised of 3 parts: a 28-week open-label, dose-finding stage, a 12-week double-blind randomized withdrawal phase in which patients continue on rusfertide or receive placebo, and a long-term extension in which all patients receive rusfertide. In the dose-finding stage, all study participants start at rusfertide 20 mg subcutaneously, with subsequent dose titration to achieve and maintain a HCT <45%. Rusfertide was administered in addition to any prior cytoreductive therapy. The current post-hoc analysis was undertaken to examine the influence of demographic and baseline disease and treatment characteristics on the effective rusfertide dose following dose titration. The impact of concomitant cytoreductive therapy with TP on the rusfertide treatment emergent adverse event (TEAE) profile was also examined. Patients were considered evaluable for this analysis if they completed the Part 1 dose-finding stage of the study.
Results
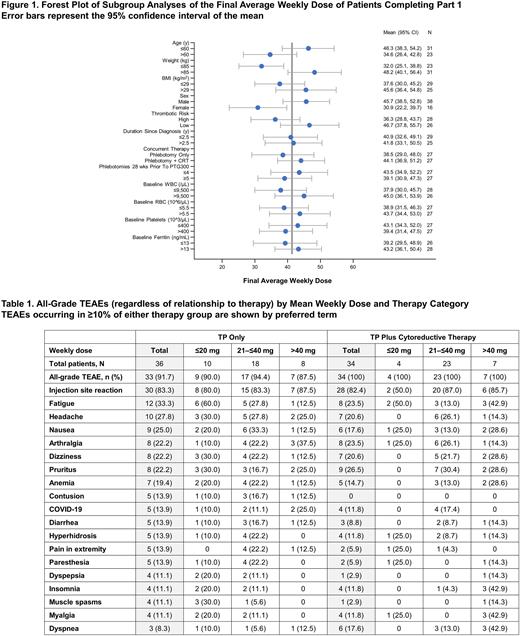
Among the 54 evaluable patients who completed Part 1 of the REVIVE study, the mean (SD) final weekly rusfertide dose was 41.3 (21.2) mg and there were no substantial differences in dose between subgroups defined by age, thrombotic risk, time since diagnosis, number of pre-study phlebotomies, concurrent treatment with cytoreductive therapy, baseline ferritin, and baseline white blood cell (WBC), red blood cell (RBC), or platelet counts (Figure 1). Average weekly rusfertide dose in female patients (30.9 mg) was observed to be lower than male patients (45.7 mg). Patients with reduced body weight (≤85 kg) appeared to require a lower average weekly rusfertide dose (32.0 mg) than those with body weight >85 kg (48.2 mg). Both groups required a lower dose than the overall group of patients who completed Part 1 (41.3 mg).
The mean (SD) final weekly rusfertide dose was similar in patients receiving TP only and TP plus cytoreductive therapy (38.5 [24.0] mg and 44.1 [18.0] mg, respectively). In the TP only cohort, there appeared to be a trend towards a lower average weekly rusfertide dose in patients with a baseline RBC count ≤5.5 x 106/µL, high thrombotic risk, female sex, weight ≤85 kg, and age >60 years. In patients receiving TP plus cytoreductive therapy, only those with body mass index ≤29 kg/m2 appeared to require a lower mean rusfertide dose (36.0 mg) compared with the overall group of patients receiving TP plus cytoreductive therapy who completed Part 1 (44.1 mg). The TEAE profile, including injection site reactions, was similar among patients receiving rusfertide ≤20 mg, 21-40 mg or >40 mg with TP only or TP plus cytoreductive therapy (Table 1). Grade 3 TEAEs in the TP only group were atrial fibrillation (n=1), gastroenteritis (n=1), peripheral sensory neuropathy (n=1), and pulmonary embolism (n=1). Grade 3 TEAEs in the TP plus cytoreductive therapy group were syncope (n=2), squamous cell carcinoma of the skin (n=1), acute myeloid leukemia (n=1), intestinal obstruction (n=1), and peripheral artery aneurysm (n=1). The syncope TEAEs occurred in patients with a prior history of syncope. All patients experiencing grade 3 TEAEs continued on study treatment except for the patient with an aneurysm and the patient with a pulmonary embolism. The pulmonary embolism was asymptomatic and incidental. There were no grade 4 or 5 TEAEs.
Conclusions
These data show that the average clinically effective rusfertide dose following dose titration is approximately 40 mg weekly. The rusfertide dose was generally consistent across the subgroups examined, suggesting that no a priori dose adjustment is necessary. However, smaller patients may require a lower average weekly dose. There was no clear or consistent increase in toxicity whether rusfertide was administered with TP only or with concomitant cytoreductive therapy.
Disclosures
Gerds:CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Imago BioSciences: Research Funding; Kratos Pharmaceuticals: Research Funding; Accurate Pharmaceuticals: Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Gotlib:BMS: Research Funding; PharmaEssentia: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kartos: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Deciphera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cogent Biosciences: Consultancy, Research Funding; Allakos: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Palmer:PharmaEssentia: Consultancy, Research Funding; Protagonist: Consultancy; CTI BioPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Bose:AbbVie: Consultancy; Pfizer: Research Funding; Telios: Research Funding; Astellas: Research Funding; NS Pharma: Research Funding; Disc Medicine: Research Funding; Promedior: Research Funding; Karyopharm: Consultancy; Cogent: Honoraria, Research Funding; Kartos: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Honoraria, Research Funding; Ionis: Research Funding; Pharma Essentia: Honoraria; Sierra Oncology (now GSK): Consultancy; Novartis: Honoraria; BMS: Consultancy, Research Funding; Blueprint Medicines Corporation: Honoraria, Research Funding; CTI BioPharma: Honoraria, Research Funding; Incyte: Honoraria, Research Funding. Ginzburg:Repare: Research Funding; Dexcel: Consultancy; Ionis: Consultancy; Takeda: Consultancy; Protagonist: Consultancy, Research Funding. Valone:Protagonist Therapeutics: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company. Modi:Protagonist Therapeutics: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Khanna:Protagonist Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. O'Connor:Protagonist Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Gupta:Protagonist Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Hoffman:Abbvie: Other: Chair DSMB, Research Funding; Silence Therapeutics: Consultancy; Novartis: Other: Chair DSMB; Protagonist Therapeutics: Consultancy; Turning Point: Research Funding; Ionis: Consultancy; Repare: Research Funding; Novartis: Research Funding; Scholar Rock: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.