Abstract
INTRODUCTION In allogeneic hematopoietic stem cell transplantation (Allo-HSCT), the Clo-Baltimore (CloB) regimen is a variant of the Baltimore reduced-intensity conditioning (RIC) regimen that uses clofarabine in replacement of fludarabine and high-dose post-transplant cyclophosphamide (PTCY). This regimen provides good survivals for patients (pts) with myeloid malignancies (Chevallier et al., Oncotarget 2018). Yet, graft-versus host disease (GVHD) remains a major cause of morbidity and mortality in Allo-HSCT recipients. The administration of anti-thymocyte globulin (ATG) is a standard method to prevent GVHD in matched Allo-HSCT. Here, we retrospectively investigated whether the addition of ATG to CloB conditioning with PTCY was beneficial in terms of GVHD rates.
METHODS This retrospective study included all consecutive Allo-HSCT adult recipients with myeloid malignancies transplanted between March 2014 and February 2022 in our hospital after CloB+/-ATG conditioning regimen. CloB consisted in clofarabine 30 mg/m²/day (d) from d-6 to d-2, cyclophosphamide 14.5 mg/kg/d on d-6 and d-5, and cyclophosphamide 50mg/kg/d on d+4 and d+5 after Allo-HSCT. In CloB+ATG, pts additionally received ATG 2.5mg/kg/d on d-2. GVHD prophylaxis consisted in the administration of cyclosporine A and mycophenolate mofetil to all patients. Graft source was exclusively peripheral blood stem cells. We determined overall (OS), disease-free (DFS) and GVHD relapse-free (GRFS) survivals, as well as non-relapse mortality (NRM) and relapse incidences.
RESULTS Sixty-six pts (median age 61 years old, range 32-74) were included, with 36 pts treated with CloB conditioning between 2014 and 2017, and 30 with CloB+ATG between 2019 and 2022. Between the two periods other conditioning regimens were used in our Department.
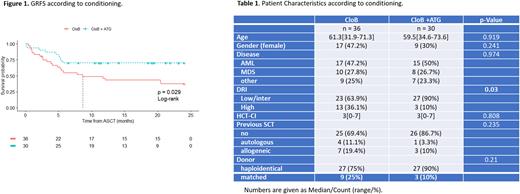
Diagnoses comprised acute myeloblastic leukemia (AML, n=32), myelodysplastic syndromes (MDS, n=18), myeloproliferative neoplasms (MPN, n=5), or MDS/MPN (n=10). The disease risk index (DRI) was low in 7 (11%) pts, intermediate in 43 (66%), and high in 15 (23%). The median HSCT-specific comorbidity index was 3 (range 0-7). Donors were haploidentical in 54 (82%) cases, and matched in 12. In the whole cohort, 18-months(m) OS, DFS and GRFS were 71±6%, 65±6%, and 55±6%, and 18-m NRM and relapse incidences were 14±4% and 21±5% respectively.
A higher proportion of high DRI was observed in the CloB group (36 vs 10%, p=0.03). The median follow-up was 68 months (m) in the CloB cohort and 19.5 m in the CloB+ATG cohort. These two groups were otherwise comparable (Table 1).
A lower proportion of acute GVHD was observed in the CloB+ATG group (23.3 vs 50%, p=0.05), with no statistical differences in terms of chronic GVHD (p=0.49). Of note, no extensive chronic GVHD was observed in the CloB+ATG group (vs 11% in CloB patients, p=0.17)
In multivariate analysis, the addition of ATG to CloB conditioning was associated with a significant increase in 18-m GRFS (70±8% vs 43±8%, HR 0.38 [95%CI 0.17-0.85], p=0.02; Figure1). No differences were observed in terms of OS (HR 0.74, p=0.51), DFS (HR 0.58 p=0.22), NRM (HR 0.84, p=0.79), relapse rate (HR 0.51, p=0.25), median time of neutrophil (>1 109/L) and platelet (> 50 109/L) recovery (respectively 19d [range 12-27] vs 18d [8-32], p=0.69, and 36d [22-471] vs 31d [12-134], p=0.09).
The results were similar when considering only patients with a haploidentical donor (n=54), with a benefit in terms of GRFS in the ATG group in multivariate analysis: HR 0.30 (95%CI 0.12-0.73; p=0.008).
CONCLUSION Addition of ATG to the Clo-Baltimore RIC regimen with PTCY improved GRFS rates in this cohort without increasing infection-related mortality nor relapse rate in pts with myeloid malignancies and matched or haplo-donors. This strategy should be more widely implemented.
Disclosures
Chevallier:Pfizer: Research Funding; Incyte: Research Funding; Takeda: Honoraria; Jazz Pharmaceuticals: Honoraria; Abbvie: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.