Abstract
The complete risk profile of CD19+ CAR T-cells could not been defined in the relatively small initial trials leading to approval of CAR T-cell products; and therefore authorities obliged CART-centers to report toxicity data on commercial CART products with the EBMT registry in Europe. Currently, there is emerging evidence demonstrating both short-term and medium-term effects, which were in part unknown at the time of regulatory approval.
Our study aimed at investigating (non-CRS and non-neurotoxicity) organ complications of commercially available CD19 CAR-T cell products in adult patients with LBCL. Data on organ toxicities were reported with information on occurrence, time of onset and grading of renal, cardiac, hepatic, gastro-intestinal, skin as well as pulmonary toxicities are reported. The current analysis focused on ≥ grade 3 (CTC-AE) toxicities. The frequency of ≥ grade 3 complications occurring in the first 100 days was assessed for the following organs: kidney, heart, liver, gut, skin and lungs. The time from CAR-T cell infusion to onset of each complication along with the grade of the complication were also described. Additional study endpoints were Overall Survival, Progression-Free Survival, Relapse Incidence and Non-Relapse Mortality.
We identified 390 patients with an available full data set on complications needed for this study. Median age was 61.4 years (range 18.7-81) and 62% were male. Karnofsky performance score was 90% or higher in 61% of the patients. Most patients received CAR-T cells without having a previous transplantation (77%). Disease status before CAR-T cell therapy was mainly chemorefractory/progressive disease in 80%. Most patients had received either two (27%) or three (37%) previous lines of therapy. However, 26% had four or more lines of previous therapy. Patients received mainly Axi-Cel (n = 255, 65%) or Tisa-Cel (n = 135, 35%).
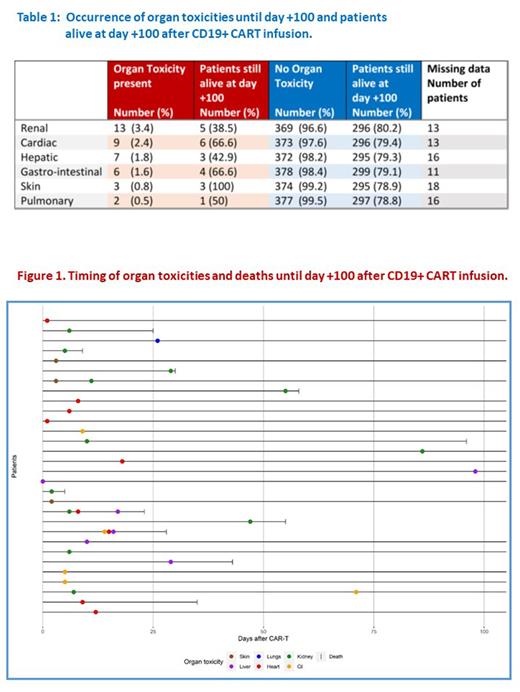
Overall, the frequency of severe (≥ grade 3) organ toxicities during the first 100 days after CAR-T was low. The occurrences of ≥ grade 3 (CTC-AE) severe toxicities in the different organs are given in Table 1. Severe renal toxicity occurred most frequently (3.4%) followed by severe cardiac, hepatic and gastro-intestinal toxicities (2.4%, 1.8% and 1.6% respectively). Our data suggests that patients with these severe complications might have a lower chance to be alive at day +100 after CAR-T cell therapy as compared to patients without organ complications. However, the absolute numbers of patients with severe organ toxicities were too low to perform more advanced statistics. The majority of severe organ complications occurred within three weeks after CAR-T cell therapy. Nevertheless, some renal, hepatic and gastro-intestinal complications occurred later between day+30 and day+100. Timing of organ toxicities is visualized in Figure 1.
Non-relapse mortality in patients with available data on organ toxicities was 2.7% [1.4-4.7; 95% CI] at 3 months and 4.2% [2.5-6.7; 95% CI] at one year after CAR-T cell therapy. The by far most frequent cause of death was relapse of LBCL (81%). The most frequent causes of non-relapse mortality were non-infectious organ toxicities (7% of total deaths) and infections (6% of total deaths). Overall survival was 82.2% [78.5-86.1; 95% CI] at 3 months and 51.6% [46.6-57.3; 95% CI] at one year. Progression-free survival was 64.1% [59.4-69.2; 95% CI] at 3 months and 32.4% [27.8-38.8; 95% CI] at one year. Finally, relapse incidence was 33.2% [28.4-38; 95% CI] at 3 months and 63.4% [58-68.3; 95% CI] at one year after CAR-T cell therapy.
In summary, we show a low frequency of severe organ complications in adults after CAR-T cell therapy for LBCL in the real-world European data.
Disclosures
Penack:Incyte: Research Funding; SOBI: Membership on an entity's Board of Directors or advisory committees; Shionogi: Membership on an entity's Board of Directors or advisory committees; Priothera: Membership on an entity's Board of Directors or advisory committees, Research Funding; Omeros: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Equillium Bio: Membership on an entity's Board of Directors or advisory committees; Therakos: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Koenecke:Novartis: Membership on an entity's Board of Directors or advisory committees. Yakoub-Agha:Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Janssen: Honoraria; Bristol Myers Squibb: Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Dreger:Novartis: Honoraria; Kite: Honoraria. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Bloor:Janssen: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Grant and personal fees, Speakers Bureau. Ganser:Novartuis: Consultancy; Jazz Pharmaceuticals: Consultancy; Celgene: Consultancy. Wulf:Novartis: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau. Novak:Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pierre Fabre: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Ideogen: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; H & O Communication: Honoraria. Moiseev:Takeda: Honoraria; Jazz: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Janssen: Honoraria. Schoemans:Incyte, Janssen, Novartis , Jazz Pharmaceuticals, Takeda: Other: Personal Fees; Belgian Hematological Society (BHS): Other: Fees paid to institution; Novartis and the BHS (Belgian Hematological Society): Research Funding; serEBMT, EUPATI (the European Patient academy): Other: serves regularly as a volunteer for EBMT and occasionally for EUPATI (the European Patient academy). Basak:Amgen: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria; Human Biome Institute: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Saventic Health: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Chabannon:EBMT: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Speakers Bureau; BELLICUM PHARMACEUTICALS: Membership on an entity's Board of Directors or advisory committees; BMS/CELGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN PHARMACEUTICALS: Membership on an entity's Board of Directors or advisory committees; TERUMO BCT: Speakers Bureau; SANOFI SA: Honoraria, Research Funding, Speakers Bureau; GILEAD: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MILTENYI BIOTECH: Research Funding; FRESENIUS KABI: Research Funding. Sureda:JANSSEN: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria; ROCHE: Consultancy, Honoraria; TAKEDA: Consultancy, Honoraria, Speakers Bureau; MSD: Honoraria; BMS: Consultancy, Honoraria; SANOFI: Consultancy, Honoraria; GILEAD: Consultancy. Glass:Roche: Honoraria; Gilead: Honoraria; Abbvie: Honoraria; Roche: Research Funding; Miltenyi: Honoraria; Novartis: Honoraria; BMS: Honoraria; Riemser: Research Funding; JAZZ: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.