Abstract
Adoptive CAR T cell therapy has revolutionized the treatment of relapsed/refractory hematopoietic malignancies. However, the autologous nature of the treatment imposes manufacturing constraints that delay chimeric antigen receptor (CAR) T cell therapy availability, may result in production failure, and are costly. The development of allogeneic, "off-the-shelf” CAR T cells aims to resolve some of these challenges. However, due to the risk of graft-versus-host disease (GvHD) with allogeneic immune cells, careful donor-matching and T cell receptor (TCR)-specificity selection is required. Alternatively, genome editing may be used to eliminate TCR expression.
Induced pluripotent stem cells (iPSCs) are an attractive source for allogeneic, "off-the-shelf” cellular immunotherapy. We have previously shown that T cell-derived iPSCs (TiPS) can provide a self-renewing source for in vitro CAR T cell production (Themeli, Nat Biotechnol, 2013), resulting in a homogenous CAR+TCR+ TiPS-derived T (iT) cell population. However, in vitro differentiation yields iT cells with suboptimal features, generating cells that eschew the CD4+CD8αβ+ double-positive (DP) stage and divert towards a CD8αα innate-like phenotype. We have shown that premature expression of the T cell receptor (TCR) causes this diversion towards an innate-like phenotype. This can be partially averted by disabling the TCR but does not allow for maturation of the cells to a CD8αβ single-positive stage due to the inability to effect positive selection through TCR engagement (van der Stegen, Nat Biomed Eng, 2022).
We have demonstrated that in the absence of a TCR, careful control of CAR expression timing and calibrated signaling strength can uniquely drive adaptive iT cell differentiation to the CD4+CD8αβ+ DP stage. CAR engagement at the DP stage subsequently facilitates maturation to CD8αβ single positive T cells, substituting for the TCR. The resulting TCR-CAR+ CD8αβ iT cells are similar to healthy-donor peripheral blood-derived CD8αβ T cells in displaying CD19 antigen specificity and dose-dependent cytotoxicity, as well as achieving effective tumor control in a systemic mouse model of leukemia, but do not cause GvHD due to complete TCR ablation (van der Stegen, Nat Biomed Eng 2022).
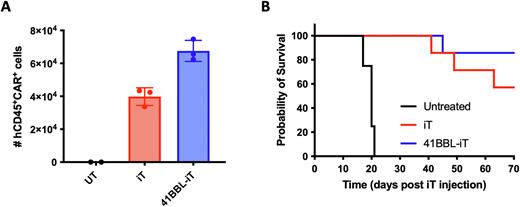
Although the resulting TCR-CAR+ iT cells are similar to their CD8+ peripheral blood counterparts, the cells require higher treatment doses, and exogenous cytokine support to achieve that result. To further increase the potency of TCR-CAR+ iT cells and remove the need for exogenous cytokine support, we enhanced iT cell function through engineering in enhanced costimulatory support. When overexpressing 4-1BBL in TCR-CAR+ iT cells, in vitro proliferation upon weekly antigen restimulation increased compared to the parental iT cells relying on solely CD28-based costimulation through the CAR. In a xenograft tumor model of disseminated leukemia, 41BBL+ TCR-CAR+ iT cells persist longer in the bone marrow (Figure 1A, measured 12 days after 4x106 iT cell injection), resulting in improved in vivo survival rates (Figure 1B).
Collectively, we show that CAR expression can drive adaptive T cell maturation in absence of a TCR, facilitating large-scale development of antigen-specific allogeneic CD8αβ iT cells devoid of allo-reactive potential and GvHD, and that TCR-CAR+ iT cell persistence and function can be further increased through developmentally regulated complementary costimulation.
Figure 1. Increased persistence and function of 41BBL-iT cells. a) Bone marrow counts of human CD45+ CAR+ iT cells 12 days post iT cell injection. b) overall survival of mice treated with 4x106 iT cells 3 days post 1x105 Nalm6 injection.
Disclosures
van der Stegen:Fate Therapeutics Inc.: Research Funding. Petrovic:Fate Therapeutics Inc.: Research Funding. Lindenbergh:Fate Therapeutics Inc.: Research Funding. Diop:Fate Therapeutics Inc.: Research Funding. Alexeeva:Fate Therapeutics Inc.: Research Funding. Xie:Fate Therapeutics Inc.: Research Funding. Clarke:Fate Therapeutics: Current Employment. Valamehr:Fate Therapeutics: Current Employment. Rivière:Akron Biotechnology, LLC: Other: Provision of Services; Centre for Commercialization of Cancer Immunotherapy: Other: Provision of Services; Fate Therapeutics: Current equity holder in publicly-traded company, Other: Provision of Services, Patents & Royalties; FloDesign Sonics: Current equity holder in private company, Other: Provision of Services; Juno Therapeutics: Patents & Royalties; Mnemo Therapeutics: Current equity holder in private company, Other: Provision of Services (uncompensated), Patents & Royalties; The Georgia Tech Research Corporation (GTRC): Other: Provision of Services (uncompensated). Sadelain:Atara Biotherapeutics: Consultancy, Patents & Royalties, Research Funding; Fate Therapeutics: Research Funding; Mnemo Therapeutics: Research Funding; Takeda Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.