In this issue of Blood, Petersdorf et al demonstrate a new paradigm for hematopoietic cell transplantation (HCT) donor selection that has an impact on the risk of relapse of malignant disorders.1
Over the last decade, advances in molecular and cytogenetic testing have identified high-risk patients with malignant disorders who would benefit from allogeneic HCT. However, disease relapse after HCT, the most common cause of mortality after HCT, continues to be a major barrier for the success of HCT.2 The use of high-resolution and ultra–high-resolution molecular HLA typing has significantly improved the outcomes of HCT,3 and current donor selection uses sophisticated publicly available algorithms that incorporate classic HLA genes, including matching the β-chain of HLA class II antigens (HLA-DRB1, -DQB1, and -DPB1). However, matching at HLA-DQB1 has not been proved to make a clinical impact, and the role of α-chain matching, such as at HLA-DQA1, remains unclear.4-6 Because HLA-DQ heterodimers have an impact on the risk of autoimmune disorders, such as type 1 diabetes and celiac disease, determining their effect on HCT was clearly warranted.7
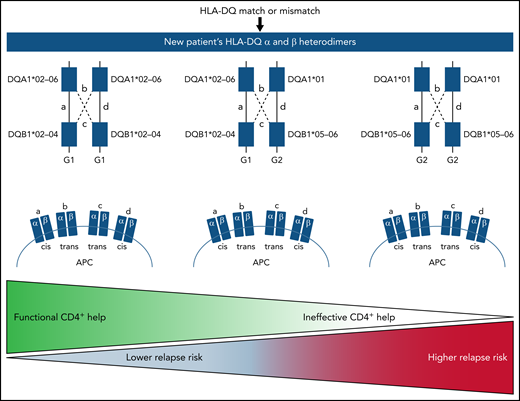
The authors evaluated the clinical implications of HLA-DQα and DQβ heterodimers after allogeneic HCT.1 The study involved 5164 HLA-DQ–matched and 520 HLA-DQ–mismatched patients and transplant donors. The authors defined heterodimer group 1 (G1) and group 2 (G2) genotypes based on the DQα and DQβ pairings. Trans-association of DQα and DQβ chains is possible for genes from the G1 and G2 genotypes, but not between G1 and G2 genotypes, meaning that a person may have up to 4 unique HLA-DQ molecules and thus up to 3 opportunities for HLA-DQ mismatches. These associations increase the diversity of molecules and therefore the HLA-DQ peptide repertoire, which in turn enhances CD4+ T-cell function. First, the authors evaluated the impact of G1 and G2 genotypes after HLA-matched transplantations and found that the presence of the G2 genotype was associated with higher rates of relapse and decreased disease-free survival. In fact, the risk increased with increased numbers of G2 genotypes; thus, both the number and nature of HLA-DQ molecules influenced the risk of relapse after HLA-matched HCT. The authors then studied the heterodimers in the HLA-DQ mismatched setting. The study again found that the number and kind of mismatched molecules had an impact on the risk of relapse after HCT. Therefore, both matched and mismatched G2 molecules increased risk of relapse after allogeneic HCT. These data are summarized in the figure.
Proposed findings for the heritance pattern and clinical implication of a patient’s HLA-DQ genotype in hematopoietic cell transplantation. APC, antigen-presenting cell.
Proposed findings for the heritance pattern and clinical implication of a patient’s HLA-DQ genotype in hematopoietic cell transplantation. APC, antigen-presenting cell.
The data presented by Petersdorf et al represent a novel paradigm for HLA-DQ, which distinguishes it from other class II antigens. This paradigm has the potential to impact transplantation outcomes for both matched and mismatched patients. HLA typing can be expanded to include additional information regarding HLA-DQ heterodimers, and more attention could be placed on HLA-DQ matching when selecting a donor. For HLA-matched transplants, patients with G2 genotypes who have high-risk disease might benefit from alternative strategies to reduce risk of relapse, such as discontinuing immunosuppressives sooner. When relying on a mismatched transplant, limiting the number of mismatched G2 genotypes could have an impact on the risk of relapse. Many patients, especially minorities, do not have the luxury of multiple donor options, but these findings could help select between 2 equally matched donors. Could a patient with very–high-risk disease benefit more from a mismatched G1 donor rather than a matched G2 donor?
The biological rationale for why the presence of the G2 genotype is associated with higher relapse rates is not obvious and warrants further investigation. There was no association of the G2 genotype and graft-versus-host disease (GVHD) in either matched or unmatched transplants. However, for malignant disorders, HCT is the most validated immunotherapy because of the graft-versus-tumor (GVT) effect, and it has been tethered to GVHD; these have been difficult to isolate from each other. The fact that HLA-DQ heterodimers have an impact only on GVT suggests that this could be a preemptive method for separating GVT and GVHD. Furthermore, these data also suggest that an exogenous source of help from CD4+ T cells provided through the allogeneic graft or at large through a cellular therapy may be the best way to reverse exhaustion of endogenous tumor-specific CD8+ T cells and revive antitumor immunity, as has been shown recently after haploidentical transplantation using posttransplant cyclophosphamide.8 HLA donor selection algorithms can now be updated to incorporate this new information to better our patients’ outcomes.
The next frontier is to dissect the immunologic role of the G2 genotype after HCT, which may provide insights into the unique biology of GVT. Once this concept is better understood, the strategy can be applied to HLA haploidentical transplants. This newly gained knowledge may have implications for cellular therapies beyond HCT.
Conflict-of-interest disclosure: The authors declare no competing financial interests.