Abstract
Glanzmann thrombasthenia (GT) is a rare inherited platelet function disorder caused by a quantitative and/or qualitative defect of the αIIbβ3 integrin. Pregnancy and delivery are recognized risk periods for bleeding in women with GT. The newborn may also be affected by fetal and neonatal immune thrombocytopenia induced by the transplacental passage of maternal anti-αIIbβ3 antibodies, which can lead to severe hemorrhage and fetal loss. Pregnancy in women with GT thus requires a multidisciplinary approach, including prepregnancy counseling and a treatment plan for delivery for both the mother and child. In this article, we summarize the current knowledge on pregnancy in women with GT and describe how we manage this severe platelet disorder in our clinical practice.
Introduction
Glanzmann thrombasthenia (GT) is a rare, inherited platelet function disorder that is caused by a quantitative and/or qualitative defect of the αIIbβ3 integrin, resulting in the absence of platelet aggregation.1 The ITGA2B gene encodes for the αIIb subunit and the ITGB3 gene for β3.2,3 The subsequent hemostatic failure is characterized by spontaneous and trauma-related mucocutaneous bleeding, with variable expression ranging from easy bruising to fatal hemorrhage.4
GT has been classified into 3 subtypes. Types I and II show quantitative abnormalities, with αIIbβ3 either absent or present only in trace amounts (<5%, type I) or at levels varying from 5% to 20% of those in normal donors (type II).5 Variant GT (also referred to as type III) is characterized by qualitative abnormalities that prevent function while allowing reduced or even normal platelet surface expression of αIIbβ3.
Generally, in cases of minor bleeding, local measures and antifibrinolytics are implemented first, while platelet transfusions are used to control or prevent life-threatening blood loss.6 They can, however, become ineffective due to the occurrence of antibodies directed against HLA class I or αIIbβ3 integrin.7-9 Antiplatelet antibodies may also cause acute transfusion reactions and impact maternal and fetal/neonatal outcomes. Activated recombinant factor VII (rFVIIa) provides an alternative treatment for patients with GT who develop platelet transfusion refractoriness, regardless of whether it is associated with antiplatelet antibodies.
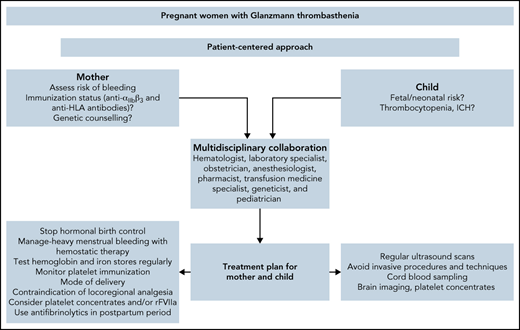
The World Foundation of Hemophilia 2019 survey found a total of at least 1832 women with GT of all ages worldwide.10 These women often have difficulty achieving pregnancy due to the recurrence of acute severe uterine bleeding when long-term hormonal therapy is stopped. The limited number of women with GT makes pregnancy and delivery a rare situation, preventing clinical trials that could provide a high level of evidence. Thus, most current medical knowledge concerning pregnancy in women with GT relies on descriptive case reports or small series.11,12 Because of the need for expertise, women should be referred to specialized hospitals with reference centers that apply a multidisciplinary approach involving a hematologist, a laboratory specialist, an obstetrician, an anesthesiologist, a pharmacist, a transfusion medicine specialist, a geneticist, and a pediatrician.13
In this paper, we describe how we currently manage women with GT before, during, and after pregnancy, presenting cases that enable us to highlight the features and dilemmas encountered when facing this challenging situation in clinical practice.
Prepregnancy period
Characterization of GT
Because GT is a severe platelet function disorder, the first signs of bleeding usually appear early in life.14 GT is readily identifiable by platelet function testing, and a lack of platelet aggregation in response to all agonists, such as adenosine 5′-diphosphate, collagen, epinephrine, or thrombin (except ristocetin), is unique for this disease.2 Flow cytometry and/or western blot analysis allow identification and quantification of the αIIbβ3 deficiency.2 Screening for the pathogenic variants of the ITGA2B and ITGB3 genes is essential for genetic counseling.3
Monoclonal antibody–specific immobilization of platelet antigens (MAIPA) is still considered the reference method to evaluate the presence of anti-αIIbβ3 antibodies in patients with GT.15 However, MAIPA is neither quantitative nor well standardized, and it cannot differentiate between antibodies that inhibit, or do not inhibit, platelet function. Based on the 2 largest series published so far, the estimated prevalence of anti-αIIbβ3 immunization probably ranges from 20% to 30%.3,7,16 Most patients with GT with immunization against αIIbβ3 have type I disease,17 and formation of anti-αIIbβ3 antibodies is rarely reported in other forms. The type of pathogenic variants affecting the ITGA2B or ITGB3 genes might also influence the risk of developing antibodies.7 The literature indicates that most of the pathogenic variants known to be associated with anti-αIIbβ3 antibodies are homozygous or compound heterozygous for premature termination variants.18,19 Furthermore, little is known about other potential risk factors, such as family history of antiplatelet antibodies, polymorphisms of genes involved in immune response, or type and amount of transfused platelets.
Table 1 summarizes the investigations required for initial characterization and evaluation of women with GT prior to pregnancy.
Prepregnancy counseling
Pregnancy is a recognized risk period for women with GT (Figure 1).8,11,12,20-22 Even for women who had received prophylaxis against postpartum hemorrhage (PPH), the reported prevalence of hemorrhages at delivery is high (40% to 50%), although studies do not always detail blood loss volume or manner of assessment or whether it occurred pre-, peri-, or postnatally.11,12
Pregnancy should also be closely monitored for anti-αIIbβ3 immunization. In a systematic review, Siddiq et al reported the management and outcomes of 40 pregnancies (including 1 twin pregnancy) of 35 women with GT, resulting in 38 live births.11 There were 3 fetal losses, 2 due to Intracranial Hemorrhage (ICH) at 24 and 31 weeks of gestation, and 1 miscarriage at 9 weeks. One neonatal death occurred 2 days after preterm birth by cesarean delivery at 28 weeks of gestation for reduced fetal movements shortly after amniocentesis. Maternal anti-αIIbβ3 antibodies were present in all 4 of these pregnancies.11 Neonatal thrombocytopenia was also reported in 3 of 38 cases, 1 with a platelet count of 35 × 109/L, associated with cephalohematoma and conjunctival hemorrhage. Again, all 3 mothers had anti-αIIbβ3 antibodies.11 Similarly, Barg et al, in a tertiary center, recently showed that 3 out of 9 newborns of 5 immunized GT women had severe thrombocytopenia.20
On the other hand, a recent survey did not provide evidence that maternal anti-HLA class I antibodies are a strong risk factor for fetal and neonatal alloimmune thrombocytopenia,23 except in rare cases.24 However, this study was not specific to GT, and anti-integrin antibodies may have different pathological effects.
Interestingly, Wihadmadyatami et al also described immunization against αVβ3 in a patient with type I GT.25 That is, β3 associates with αv to function as a vitronectin receptor, essentially on endothelial cells. The authors demonstrated that these antibodies could disturb endothelial cell adhesion to vitronectin, trigger endothelial cell apoptosis, and interfere with endothelial tube formation. Moreover, antiendothelial αvβ3 antibodies are a major cause of ICH in fetal/neonatal alloimmune thrombocytopenia.26 We recently found that patients with Gypsy GT who failed to express αIIbβ3 on the platelet surface can also develop such anti-αvβ3 antibodies.27 Although further data on this type of immunization are required, identification might eventually predict fetal/neonatal ICH development in immunized women with GT.
Accordingly, women with GT and their partners should be warned regarding the risk of maternal and fetal/neonatal complications, especially ICH related to severe fetal/neonatal thrombocytopenia. Pregnancy should be considered high risk in women with evidence of current immunization against anti-αIIbβ3 and/or platelet transfusion refractoriness and should be discouraged, particularly in women with a history of either life-threatening PPH or fetal/neonatal ICH or when the anti-αIIbβ3 antibody levels is high (defined, in our laboratory, by a ratio of optical density more than a 2-fold increase compared with controls) (Figure 1). Women with a lower risk of complications should also be informed that their safety and that of their infant will require close and demanding follow-up throughout pregnancy, at delivery, and postpartum. Preconception counseling is particularly challenging in nulliparous women who, by definition, have not experienced a natural course of pregnancy. Moreover, despite repeated preconception visits, some women with GT are then seen already pregnant, regardless of the risks previously explained. Finally, when a pregnancy is planned, hemoglobin level, iron status, or nasal and oral health should be evaluated before conception to prevent avoidable complications during pregnancy.
Management of heavy menstrual bleeding during the prepregnancy period
Case 1
Case 1 involves a 35-year-old woman seen in our hematology department for management of GT. Her platelet disorder was diagnosed at birth following diffuse ecchymosis. Western blot analysis showed classic type I GT with trace amounts of αIIb and residual expression of β3, whereas molecular analysis revealed that she was compound heterozygous in the ITGA2B gene for (1) a splicing defect (c.2095-19T>A; classified as “uncertain significance” according to the American College of Medical Genetics classification, which we used to assess all gene variants herein); this variant was not found in a population database and is predicted to create a cryptic splicing acceptor site with 5 different computational predictive models; and (2) a nonsense variant (c.2770C>T/p.Gln924*; classified as “pathogenic”). At the age of 4, the patient had a massive tracheal hemorrhage that was managed with platelet concentrates and red blood cells. After this episode, testing for anti-αIIbβ3 antibodies was weakly positive, although she was negative for anti-HLA class I antibodies. According to the data on outcomes after surgical interventions published in the international Glanzmann Thrombasthenia Registry, only 8 of 43 (18.6%) of the immunized patients had antiplatelet antibodies directed against both the integrin and HLA system. This finding suggests that these antibodies are not frequently associated,16 perhaps because each type of immunization induces different responses.
Case 1 wanted to become pregnant and thus, in agreement with our team, stopped oral contraception. This exposed her to a high risk of bleeding during menstrual periods, a common problem with GT.22,28,29 Women of reproductive age are particularly vulnerable to heavy menstrual bleeding (HMB), with estimated incidences ranging from 74% to 98%.30
In our patient, HMB with blood clots responded partially to tranexamic acid given at weight-appropriate doses (1 g repeated every 8 hours). A high dose of intranasal desmopressin has also been used for the management of HMB in women with GT but was found to be ineffective, except in women with type II, for whom it may have some benefits.31 We planned to avoid platelet transfusions as much as possible for Case 1 because of the high risk that anti-αIIbβ3 antibodies would bring. Supportive care should also include iron replacement therapy for secondary anemia.6
Because Case 1 had not become pregnant after 12 months, she underwent a pelvic ultrasound and hysterosalpingography to explore this primary infertility. They showed intrauterine blood clots that interfered with egg implantation. Blood clots were then removed by hysteroscopy. Because recent testing for anti-αIIbβ3 antibodies showed the presence of residual immunization, this invasive procedure was performed using several doses of rFVIIa rather than platelet concentrates to avoid stimulating these antibodies. In France, rFVIIa is currently indicated for patients with GT with past or present refractoriness to platelet transfusions or when platelets are not readily available.6,7 Specific indications may differ from 1 country to another. Data from the international Glanzmann Thrombasthenia Registry show that rFVIIa is also frequently used off-label for bleeding and surgical procedures, regardless of platelet antibodies and/or transfusion refractoriness.9,16,32,33 Administration of 80 to 120 µg/kg of rFVIIa is recommended, repeated every 2 to 3 hours, and at least 3 doses should be administered to achieve hemostasis.6 rFVIIa was rated effective in >88% of surgical procedures.16
Rare cases of medically assisted reproduction have been also previously reported in women with GT to limit severe acute bleeding after interruption of hormonal treatment.34-36 These usually involved ultrasound-guided transvaginal follicle aspiration and the use of rFVIIa at standard dosages for hemostatic therapy to prevent the risk of bleeding related to the procedure.
Antenatal period
Management of miscarriage, ectopic pregnancy, or other obstetric complications
Case 1
The approach described above enabled Case 1 to become pregnant spontaneously. Unfortunately, she then miscarried at 7 weeks of gestation and underwent curettage, managed by 3 doses of rFVIIa at a standard dosage, associated with tranexamic acid.
Although the course of pregnancy does not seem to be worse in women with GT, some complications, such as spontaneous miscarriage or ectopic pregnancy, require careful management due to the related high risk of bleeding.11,12,37,38 Although data on general outcomes are sparse, Civaschi et al compared the course of pregnancy of 10 women with GT to that of the general population and found them to be similar. The rate of miscarriage plus stillbirth was 11.8% (95% CI, 1.4-36.4), and there were no preterm births.12 Finally, there is no evidence that women with GT have higher rates of spontaneous miscarriages or other antenatal obstetric complications. Nevertheless, the literature is devoid of data specifically regarding the management of these events in patients with GT. To minimize hemorrhage, we suggest surgical management by manual extraction or vacuum aspiration for pregnancy loss and systematic ultrasound verification to ensure the absence of retained products of conception. The use of misoprostol for medical treatment can be considered in some cases of early pregnancy loss. Hemostatic coverage should be preventively implemented and adapted on a case-by-case basis to limit bleeding complications.
In case of ectopic pregnancy, it seems reasonable to consider surgical management (salpingotomy or salpingectomy), and in a few cases, methotrexate therapy might be considered. However, it is crucial to underline that these proposed strategies are not based on evidence. In all cases, prompt hemostatic management, adapted on a case-by-case basis, should be carried out in consultation with hematologists to implement a clear plan for suitable hemostasis.
To our knowledge, placental vascular complications, including hypertensive disorders, fetal growth restriction, or placental abruption, do not appear to occur at higher rates in women with GT than in the general population of parturients. Thus, the use of aspirin or other antithrombotic therapy should be contraindicated unless it provides significant clinical benefits.
Antiplatelet immunization during pregnancy
Case 1
In their literature review, Siddiq et al reported anamnestic response with rising anti-αIIbβ3 antibody titers in 3 of 22 tested pregnancies.11 Various therapies directed against anti-αIIbβ3 antibodies have been reported to facilitate maternal bleeding management and include plasma exchange, immunoadsorption, corticosteroids, or intravenous immunoglobulin.39-44 In women without GT, recent recommendations for fetal and neonatal alloimmune thrombocytopenia suggest considering weekly antenatal use of intravenous immunoglobulin, associated with corticosteroids or not.45 Similarly, antenatal intervention at 12 to 16 weeks of gestation might be considered for women with GT with a previous pregnancy involving fetal/neonatal ICH related to anti-αIIbβ3 antibodies (Figure 2). For all other pregnancies of mothers with (1) a previous infant with isolated neonatal thrombocytopenia, (2) current high maternal anti-αIIbβ3 antibodies, or (3) a significant rise in anti-αIIbβ3 antibodies during pregnancy, maternal antenatal intervention should be explained and discussed with each patient at 20 to 22 weeks of gestation. Women with a low level of maternal anti-αIIbβ3 antibodies at pregnancy onset and nonimmunized patients with GT at high risk of developing antibodies should undergo regular assessments using MAIPA to identify rising antibody titers. In women at low risk of developing anti-αIIbβ3 antibodies, antiplatelet antibodies screening could be proposed at the end of the third trimester.
A few months after her miscarriage, Case 1 again became pregnant spontaneously. Due to her GT type and history of anti-αIIbβ3 antibodies, we tested for them monthly, starting in the second trimester, given the plausible risk that they would increase during pregnancy. Because testing showed no such increase, no specific treatment was initiated. Ultrasound scans were performed every month to monitor fetal growth and screen for the absence of ICH. The rest of the pregnancy was uneventful.
Genetic counseling and prenatal diagnosis
Case 2
The second patient is a 20‐year‐old woman seen in our hematology department for management of GT type II. From infancy, she repeatedly experienced easy bruising and epistaxis as prominent GT symptoms. They turned out to be controllable by local treatment and antifibrinolytics. Diagnosis of GT type II had already been established in childhood by (1) a normal platelet count, (2) a strong reduction of platelet aggregation with all agonists, except ristocetin, and (3) a flow cytometry study showing platelets with 18% to 24% residual αIIbβ3. Molecular analysis revealed that she was compound heterozygous for pathogenic variants in the ITGB3 gene (c.187C>T/p.R63C and c.505C>T/p.R169*; both variants classified as “pathogenic”). At the age of 10, due to prolonged bleeding at menarche, the patient had required progesterone and antifibrinolytics. Nonetheless, she had no history of severe bleeding requiring treatment by platelet concentrates or rFVIIa. Unsurprisingly, in the absence of blood transfusion exposure, testing was negative for anti-αIIbβ3 and anti-HLA class I antibodies.
Case 2 wanted to become pregnant. Because she was married to a cousin, we proposed screening the spouse for ITGB3 pathogenic variants to ensure that there was no risk of homozygosity in the unborn child. The husband was negative for both pathogenic variants.
Because GT is an autosomal recessive disorder, in the absence of consanguinity, newborns are obligate heterozygous carriers for ITGA2B or ITGB3 pathogenic variants. Nevertheless, GT occurs relatively frequently in certain ethnic populations with higher incidence rates of consanguinity, such as Iraqi Jews, Palestinians, or Jordanian Arabs.46,47 Molecular analysis allows identification of asymptomatic heterozygous carrier partners, who cannot be identified with certainty by phenotypic studies alone.2 We thus suggest heterozygous carrier screening in case of consanguinity and desire for pregnancy, as for Case 2. On the other hand, the probability that a non-consanguineous partner has a pathogenic variant of the same gene is slight, and the possibility of finding a variant of uncertain significance in full gene screening is nontrivial. This could create complex issues for prenatal diagnosis. For all these reasons, we do not consider systematic full gene study in this context.
Carriers should receive preconception counseling about genetic transmission, and the possibility of resorting to prenatal diagnosis should be considered. The bleeding risk associated with this procedure also requires appropriate hemostatic management.
Prenatal diagnosis has rarely been reported in patients with GT.48-51 It may be proposed in cases of consanguinity because of the risk to the offspring, given the 50% risk of an affected fetus for a woman with GT and a consanguineous heterozygous partner. Invasive prenatal diagnostic methods, such as chorionic villus sampling and amniocentesis, allow definitive diagnosis but are associated with a risk of procedure-related fetal loss that should be discussed with the couple.
Management of maternal antenatal bleeding
Case 2
Data suggest that the risk of unprovoked antenatal bleeding in women with GT is low.11,12 In any event, bleeding should first be managed by local measures and/or tranexamic acid (Figure 2). Caution is required when using antifibrinolytic therapy because data are too limited to ensure the absence of teratogenicity in early pregnancy (first trimester). Furthermore, platelet transfusions should be avoided in pregnant women with GT at high risk of developing anti-αIIbβ3 antibodies.
In Case 2, no significant events occurred before pregnancy, which began spontaneously. Recurrent epistaxis and gingival bleeding were managed by topical measures and tranexamic acid during the second and third trimesters.
Delivery and postpartum management: general principles
Delivery should be planned in referral centers that can provide a multidisciplinary team approach and have easy access to blood products, hemostatic therapies, specialized laboratory tests, pelvic arterial embolization, and a maternal intensive care unit (Figure 3).
A personal history of significant bleeding before pregnancy appears to be associated with PPH, whereas the mode of delivery (vaginal vs cesarean) does not.11,12 Accordingly, both delivery options should be considered, taking into account (1) obstetric conditions, (2) the risk of neonatal thrombocytopenia, (3) maternal bleeding history, and (4) the organization of the local department.
Epidural analgesia is categorically contraindicated, and pain should be managed with other options, such as a patient-controlled morphine pump. A clear plan should be made for delivery monitoring, including options for various potential emergencies, in consultation with the entire multidisciplinary team, including obstetric, hematology, and anesthesia teams. Some practitioners advocate avoiding instrumental delivery in a trial of labor due to the risk of neonatal thrombocytopenia and ICH. During the second stage of labor, however, when the fetus is engaged at a low or outlet pelvic station, severely abnormal fetal heart rate tracings may occur and justify expediting delivery rapidly to avoid hypoxic-ischemic encephalopathy of the newborn. In such difficult situations, instrumental compared with cesarean delivery is likely to be the procedure associated with a lower rate of morbidity for both the mother and the neonate and should be performed.52,53 Forceps are then the instrument of choice; vacuum delivery should be contraindicated because compared with forceps delivery, it may increase the rates of cephalohematomas, diffuse subcutaneous hematomas of the scalp (subgaleal hematoma), and ICH,53,54 all complications that are theoretically higher among women with GT, in case of neonatal thrombocytopenia.
The prophylactic administration of a uterotonic agent immediately after delivery is recommended for all women to prevent blood loss,54,55 especially for women with GT. Moreover, women with vaginal delivery who receive prophylactic oxytocin and 1 g of tranexamic acid after cord clamping have lower rates of provider-assessed clinically significant PPH and receive additional uterotonic agents less often.54,56-58 In addition, among women who underwent cesarean delivery and received prophylactic uterotonic agents, 1 g of tranexamic acid after cord clamping resulted in a significantly lower calculated estimated blood loss.59 In a retrospective study, tranexamic acid has also been shown to decrease PPH in women with bleeding disorders.60 These studies suggest that 1 g of tranexamic acid should be administered prophylactically immediately after cord clamping. Moreover, in cases of abnormal blood loss, early use of an additional 1 g of intravenous tranexamic acid is recommended for women with clinically diagnosed PPH, and a third 1 g dose is recommended if bleeding continues after 30 minutes.61 Nevertheless, given that previous reports have highlighted the high risk of blood loss after delivery in women with GT,12 prophylactic administration of 1 g of tranexamic acid every 8 hours might be considered in the postpartum period.
PPH is a major concern in women with GT, sometimes even in case of administration of prophylactic hemostatic treatment.11 Primary PPH (blood loss >500 mL by 24 hours after delivery) and severe primary PPH (blood loss >1000 mL during the first 24 hours after delivery) that require blood products or hemostatic therapy supports have been reported to arise in approximately one-third of pregnancies in women with GT. Secondary PPH may occur in 24% of cases at a median time of 10 days after delivery11 and may occur even up to 6 to 8 weeks postpartum. Thus, women with GT should be offered oral contraceptive therapy early after delivery to prevent hemorrhages due to the return of menstruation. Estrogen-progestins, more generally, are not recommended during the first 6 weeks after delivery.
To our knowledge, there is no specific recommendation concerning breastfeeding by women with GT and they can thus breastfeed normally. Finally, theoretically, antiplatelet antibodies may be passed on to infants through breastfeeding. However, this would likely be at low levels because transfer of IgG antibodies via breastmilk is more limited than transfer of IgA antibodies.
Managing delivery and postpartum in women with GT with anti-αIIbβ3 antibodies
Case 1
Labor began spontaneously at 39 weeks of gestation. A patient-controlled morphine pump was used for pain management. Our blood bank obtained platelet concentrates before delivery in case they were urgently needed. The patient gave birth spontaneously to a healthy girl weighing 3560 g. A first dose of 90 µg/kg−1 of rFVIIa was administered within 5 minutes after the birth, together with prophylactic oxytocin (10 IU) and 1 g of prophylactic tranexamic acid.
Just after delivery, estimated blood loss, assessed with a collector bag, was 550 mL. General anesthesia was then administered to enable manual removal of the placenta and manual examination of the uterus, in accordance with French as well as international guidelines.54,55,62 This allowed the removal of several blood clots, and intravenous therapeutic oxytocin was administered. A first-degree perineal laceration was sutured, and a higher second dose of rFVIIa (120 µg/kg−1) was administered 2 hours after the first rather than the originally scheduled 3 hours. The platelet concentrates were not administered because neither treatment failure nor severe blood loss had occurred.
The patient was then admitted to the intensive care unit, with a total estimated blood loss of 750 mL and a hemoglobin concentration of 10 g/dL. She received a final dose of 90 µgkg−1 of rFVIIa 3 hours after the second dose. Additional rFVIIa was not necessary because there was no postpartum bleeding.
Cord blood should always be tested immediately after delivery to screen for neonatal thrombocytopenia (Figure 3).11,22 Recent international guidelines on alloimmune thrombocytopenia also recommend a cranial ultrasound to screen thrombocytopenic neonates for ICH within 24 hours of delivery and immediate platelet transfusion if life-threatening bleeding is present or the platelet count is <30 × 109/L.45 A similar approach might be implemented for infants born of mothers with GT. The neonatal platelet count should be monitored regularly until normalization. In Case 1, the neonatal platelet count was normal, and no intervention was required.
Tranexamic acid was continued for 10 days and oral low-progesterone contraception was initiated. The patient was discharged home on day 4 postdelivery and developed no secondary PPH.
Managing delivery and postpartum in women with GT with low risk of anti-αIIbβ3 antibodies development
Case 2
Because the patient lived so far from our hospital, labor was induced at 39 weeks of gestation. In patients with GT, platelet transfusion may be ineffective because of competition between transfused and endogenous platelets at vascular injury, and successful management of these patients may require administration of a consistent number of platelet units.63,64 Due to the low risk of anti-αIIbβ3 immunization in this woman with type II GT, random-donor platelet concentrates containing a total of 0.5 × 1011 platelets per 7 kg of weight were preferred to rFVIIa and administered after spontaneous delivery of the neonate. The placental delivery was uneventful, and the total estimated blood loss was 400 mL. Her hemoglobin concentration was 11 g/dL after delivery, and the administration of additional platelet concentrates was unnecessary in the absence of postpartum bleeding. Tranexamic acid continued for 10 days after delivery. Low-progesterone oral contraception began, and the patient was discharged home on day 5 postdelivery.
Conclusion
GT is a disorder at high risk of bleeding that requires a multidisciplinary team approach to pregnancy management. GT typing and anti-αIIbβ3 screening or follow-up are crucial for an appropriate therapeutic approach. Because most treatment recommendations have been based on case reports or series, questions remain as to the optimal management of the mother and safe delivery of the newborn, and they need to be resolved in the future. However, the impact of anti-αIIbβ3 antibodies is huge both for mother and fetus/neonate and should prompt special attention and specific care for young girls with GT type I to prevent future pregnancy complications.
Authorship
Contribution: R.d'O. and M.F. analyzed literature data and reported GT cases studies; R.d'O., M.F., and L.S. wrote the paper.
Conflict-of-interest disclosure: R.d'O. and M.F. received fees from NovoNordisk. The remaining author declares no competing financial interests.
Correspondence: Mathieu Fiore, Laboratoire d’hématologie, Centre de Référence des Pathologies Plaquettaires, CHU de Bordeaux, Hôpital Cardiologique, Inserm U1034–Biologie des Maladies Cardio-Vasculaires, Pessac 33604, France; e-mail: mathieu.fiore@chu-bordeaux.fr.