Abstract
Real World Experience: Blinatumomab in the Treatment of Acute Lymphoblastic Leukemia in Mexico.
Introduction
Treatment of acute lymphoblastic leukemia (ALL) in adults can achieve complete hematologic remissions (CHR) of 80%, however, 50% of patients have relapse with a poor prognosis.
Minimal Residual Disease (MRD) > 0.01% is the main independent prognostic factor after induction treatment in ALL, which is why it has become the cornerstone in decision-making.
Blinatumomab is a CD19/CD3 bispecific antibody designed to redirect previously unstimulated T cells to malignant B cells and induce their destruction. The FDA approved it in 2014 in R/R ALL by demonstrating improvement in CHR and Overall Survival (OS) rates in the TOWER study. In 2018, it was approved for the treatment of ALL in CHR, with MRD + based on the BLAST study in which 78% of patients presented MRD ND at the end of the first treatment cycle.
Goals
Number of patients in CHR and MRD.
Number of adverse events during administration.
Number of patients in OS and Relapse-Free Survival (RFS).
Methods
Retrospective, cross-sectional, observational and descriptive study. The records of patients who received blinatumomab from January 2018 to May 2021 were reviewed. Pre and post blinatumomab MRD was evaluated by flow cytometry, MRD response was defined as Not Detectable (ND) or less than 0.01%.
Statistical analysis
The qualitative variables were studied in frequencies and proportions, the quantitative variables were grouped into medians and ranges. OS and RFS were determined using Kaplan-Meier survival curves with log-Rank with a significant p <0.05 value. Statistical analysis was performed with SPSS version 24.
Results
Eighteen patients were included, 66.7% (12) men and 33.3% (6) women. The mean age was 30 years. By age group, 72.2% (13) were adolescent and young adult, 22.2% (4) were adults and only 5.6% (1) were older adults.
The time in which adverse events occurred, 33.3% (6) were recorded in the first 24 h after blinatumomab, 38.9% (7) were at 24-72 h and 27.8% (5) after 72 h. Fever was the most frequent in patients 77.8% (14), neurological events 44.4% (8), such as distal tremor 38.9% (7), headache 27.8% (5), seizures 11.1% (2). Cytokine release syndrome were 27.8% (5) and infections 38.9% (7). Grade 3 adverse events were in 22.2% (4) of the patients.
Due to toxicity, it was decided to suspend the blinatumomab infusion in 33.4% (6) of the patients and in 2 of them it was definitively.
The response after blinatumomab: 77.8% (14) patients with MRD ND, 5.6% (1) presented MRD of 1x 10 -4 and only 16.8% (3) with 1x 10 -1. Hematopoietic Stem-Cell Transplantation (HSCT) was carried out in 27.8% (5) of the patients.
The current status of the patients with 72.2% (13) with CHR and MRD ND, in relapse 11.1% (2), one was after HSCT and 16.7% (3) have refractory disease.
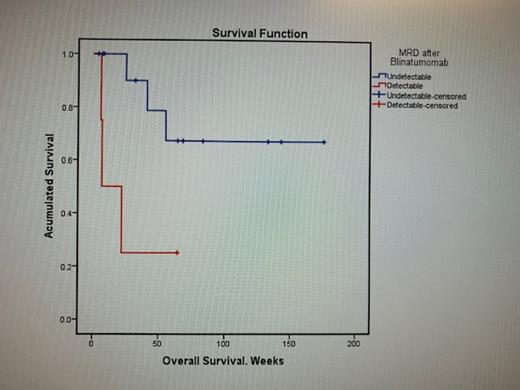
The mean OS was 37.53 months and RFS was 14.13 months. The results that were statistically significant in OS compared by Kaplan Meier curves were: relapse (X 2 = 4.59, p = 0.032), blasts <50,000 x 10 3/mm 3 (X 2=11.98, p=0.001), MRD ND after infusion of blinatumomab (X 2 = 5.4, p = 0.02).
Discussion
The response after blinatumomab infusion was: 77.8% (14) patients with MRD ND, 5.6% (1) presented MRD of 1x 10 -4 and only 16.8% (3) presented values greater than 1x 10 -1. HSCT was carried out in 27.8% (5) of the patients. These results are similar to those reported by Gökbuget in the BLAST study in 2018 where he reported 78% of MRD ND with a RFS of 23.6 months in responding patients. The most frequent adverse events were Grade 1 and 2, similar to those reported in the literature.
Conclusions
This study represents the largest series of patients in Mexico to describe the clinical characteristics and responses with the use of blinatumomab in patients with ALL. Blinatumomab is a new drug for the treatment of ALL that has proven to be effective and well tolerated the greatest benefit is observed in the context of detectable MRD, being more effective and with fewer side effects than chemotherapy.
The limitations of the study are the small number of patients and the limited access to blinatumomab.
Ceniceros: Amgen: Speakers Bureau.