Abstract
Introduction.
Acute lymphoblastic leukemia (ALL) represents approximately 52% of pediatric cancer diagnoses in Mexico and is the leading cause of death by disease among children of 5 to 14 years. The use of pharmacogenomics for the detection of germline genetic variations, which are associated with sensitivity or toxicity to chemotherapy, is one of the recent strategies to improve survival. Some mutations present in genes related to the metabolism or transport of methotrexate (MTX) have been associated with the occurrence of toxicities during treatment in pediatric acute lymphoblastic leukemia. The effect of the SNP´s rs1801131, rs1901133 in the MTHFR and rs4149058, rs4149081 in the SLCO1B1 gene have not been studied in Northwest Mexican children with acute lymphoblastic leukemia.
Patients and methods.
Eligible patients were infants less than 1 year to adolescents less than 18 years of age, diagnosed with B-ALL at the Specialties Children's Hospital of Chihuahua, Mexico. Patients were treated according to the locally protocols based on the St. Jude Children's Research Hospital Total XIIIB and Total XV protocols. We followed-up for two to four weeks after 24 hours high dose MTX (1 to 5 g/m 2) administration. Toxicity data were collected objectively from the patient's medical files and adverse effects were classified as a dichotomous variable yes/no. Genomic DNA was extracted with the Master Pure DNA purification kit (Epicentre Illumina® Company) from peripheral blood. Genotyping assays were performed by real-time polymerase chain reaction, using rhAMp® IDT® genotyping probes (Iowa, USA) and Quant Studio 3 Applied Biosystems Real-Time System Thermal Cycler (Thermo Fisher Scientific®). For statistical analysis association between MTX dose, presence of toxicity, and genetic polymorphisms was evaluated by the chi-square or Fisher's exact test. The effect sizes of the associations were estimated by the OR's from univariate logistic regressions and multivariate logistic regressions to account for the possible confounding effect of sex and age. Analyses were performed by using SAS System and IBM SPSS Statistics Base 22.0 software.
Results.
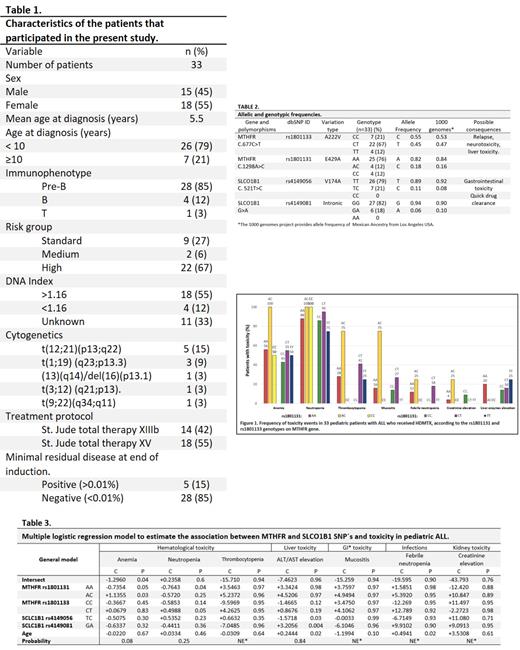
The study population demographics and clinical characteristics are summarized in Table 1. MTHFR and SLCO1B1 genotype in our patients and controls obtained from the 1000 Genomes Project database are shown in table 2. In general, a predominance of toxicity events is observed in patients heterozygous for both polymorphisms in the MTHFR gene, but not in the SLCO1B1 gene (Figure 1). Through a logistic regression analysis, we observed an association of 8% between the polymorphisms rs1801131, rs1901133, rs4149058, rs4149081 and the presence of anemia. The AC heterozygous of the rs1801131 has the strongest association with a positive coefficient (+1.1355) and when comparing AC heterozygotes with CC mutated homozygotes, an Odds Ratio (OR) of 4.64 (CI: 95%, 0.719) was observed, identifying it as a risk factor. In contrast, the homozygous AA of the same gene, presented a negative coefficient (-0.7354), thus decreasing the probability of presenting anemia in this population. The presence of neutropenia was associated in 25% with the polymorphisms rs1801131, rs1901133, rs4149058, rs4149081. The AA homozygous of rs1801131 decreased the probability of developing neutropenia (coefficient -0.7643, p=0.04) and the TC heterozygous of rs4149056 increased the probability when comparing with the wild TT homozygotes (OR of 2.91, CI: 95%, 0.496). Liver enzymes elevation was associated in 84% with the polymorphisms rs1801131, rs1901133, rs4149058, rs4149081. We found that the presence of TC heterozygote of rs4149056 decreased the probability of hepatotoxicity (coefficient -1.5718, p=0.03) and GA heterozygote of rs41419081 increased it (coefficient +3.2056, p=0.004) (Table 3). According to this statistical model, we could not analyze the association between the polymorphisms rs1801131, rs1901133, rs4149058, rs4149081 and thrombocytopenia, mucositis, febrile neutropenia or creatinine elevation.
Conclusion
Toxicity related to treatment is one of the most important causes of death among pediatric ALL patients in Mexico, the development of precision medicine through the identification of SNPs associated with the variability in our patients' response is an alternative to minimize methotrexate toxicity and maximize its benefit during its use.
No relevant conflicts of interest to declare.