Abstract
Background
Somatic mutations are an important prognostic factor in chronic myelomonocytic leukemia (CMML). However, limited data are available regarding their impact on outcomes after allogeneic hematopoietic cell transplantation (HCT). In this registry analysis conducted via the Center for International Blood and Marrow Transplant Research (CIBMTR) as well as its sample repository, we analyzed the landscape of somatic mutations in patients with CMML and their impact on post-HCT outcomes.
Methods
Using the CIBTMR database, adult patients with CMML who underwent HCT from 2001-2017 with an available specimen in the National Marrow Donor Program (NMDP) repository were identified; samples were collected prior to the start of conditioning. Patients who received umbilical cord blood, syngeneic, or haploidentical HCT or patients with disease transformation to acute myeloid leukemia prior to HCT were excluded. The primary endpoint was overall survival (OS); secondary endpoints included disease-free survival (DFS), relapse/progression, treatment-related mortality (TRM); survival outcomes were calculated from time of HCT. A mutation analysis for 131 genes recurrently mutated in myeloid disorders was performed.
Results
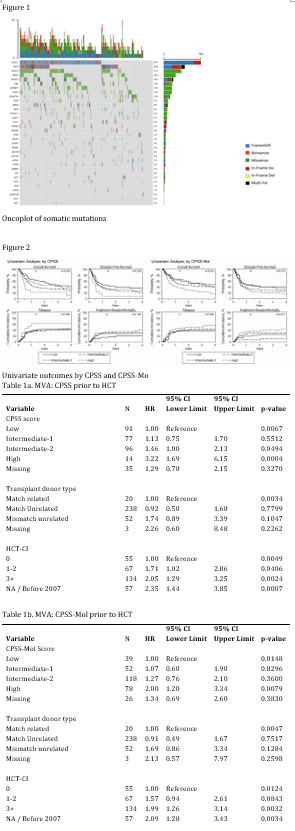
We identified 313 patients across 78 centers who met inclusion criteria with available samples. The median age was 64, and 69% were male. By World Health Organization (WHO) criteria, 53%, 20%, and 9% of patients had CMML-0, -1, and -2, respectively. By French-American-British (FAB) criteria, 76% of patients had dysplastic (MD)-CMML and 24% had proliferative (MP)-CMML. The majority (61%) had received hypomethylating therapy prior to HCT, and 76% of HCT were from a matched unrelated donor. Distribution of patients by CMML-specific prognostic scoring system (CPSS) score was 29% low, 25% intermediate-1, 31% intermediate-2, and 4% high (11% missing). Distribution of patients by clinical/molecular CPSS (CPSS-Mol) score was 12% low, 17% intermediate-1, 38% intermediate-2, and 25% high (8% missing).
Of the 313 patients studied, 290 (93%) had 1+ pathogenic mutation identified, and the median number of mutations was 3. The most frequently mutated genes were ASXL1 (62%), TET2 (35%), KRAS/NRAS (33% combined), and SRSF2 (31%); TP53 was mutated in 3% of patients (Figure 1). U2AF1 mutations were significantly correlated with MD-CMML, and ASXL1, EZH2, KIT, and SRSF2 mutations were significantly correlated with MP-CMML.
The CPSS score was correlated with OS and DFS on univariate analysis, and the CPSS-Mol score was correlated with OS and TRM (Figure 2). Neither scoring system was predictive for progression/relapse.
In multivariable analysis, as compared to a CPSS score of low, intermediate-2 (hazard ratio [HR]=1.46, p=0.049) or high (HR=3.22, p=0.0004) correlated significantly with overall survival (OS) (table 1a). When CPSS-Mol was applied, a high score (HR=2, p=0.0079) correlated significantly with OS (table 1b). In multivariable analysis, DNMT3A and TP53 mutations were associated with decreased OS (HR=1.70, p=0.0147; and HR=2.72, p=0.0042, respectively) while DNMT3A, JAK2, and TP53 mutations were associated with decreased DFS (HR=1.66, p=0.0138; HR=1.79, p=0.0293; and HR=2.94, p=0.0018, respectively). The only mutation associated with increased relapse was TP53 (HR =2.94, p=0.0201). DNMT3A was associated with increased TRM(HR=1.89, p=0.0388) and PTPN11 was associated with decreased TRM (HR=0.205, p=0.0300). Goodness of fit was calculated by Harrell's C-index for CPSS and CPSS-Mol for OS, DFS, relapse, and TRM. For CPSS, the results were 0.56, 0.55, 0.52, and 0.56, respectively while for the CPSS-Mol they were 0.57, 0.55, 0.55, and 0.58, respectively.
Conclusion
Our study is the largest analysis of CMML patients who underwent HCT with paired mutation analysis. Via registry data we have provided the mutational landscape in patients with CMML who underwent HCT and demonstrated a strong association between CPSS-Mol and transplant outcomes although without major improvement in the risk prediction beyond the CPSS.
Mei: TG Therapeutics: Research Funding; EUSA: Honoraria; Epizyme: Research Funding; BMS: Research Funding; Morphosys: Research Funding; Janssen: Honoraria; Beigene: Research Funding. Sobecks: CareDX: Membership on an entity's Board of Directors or advisory committees. Scott: Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Saber: Govt. COI: Other.