Abstract
Introduction
Carfilzomib (K or Cfz) is a widely used second-generation proteasome inhibitor for patients with multiple myeloma (MM). However, despite the numerous positive clinical results, there were only a few "real-world" studies, particularly among elderly patients. Avoiding Cfz for the elderly may be based on a potential selection bias to avoid rare but serious cardiovascular adverse events (CVAEs) associated with Cfz. Moreover, although Cfz-associated thrombotic microangiopathy (TMA) has been recently recognized, the incidence rate of TMA varied from less than 1% to 5.0%. To determine the efficacy of Cfz and occurrence of CVAEs in the elderly patients and the incidence of TMA, we reviewed 96 patients, including 28 elderly patients (aged ≥76 years) at our center. All participants or their family members provided written informed consent for inclusion in the study. The study was conducted according to the Declaration of Helsinki and approved by the ethical review board of our institute.
Methods
Cfz was administered in the same protocol as in each clinical trial. The choice of the Cfz regimen and the criteria for dose reduction, including dexamethasone use, were based on the patient's background and discretion of the treating physician.
Severe CVAEs were defined as events of grade ≥3 including heart failure, acute coronary syndrome, relative reduction in the left ventricular ejection fraction ≥20% from baseline, QT prolongation (corrected QT: ≥501 ms), and hypertension (systolic blood pressure of ≥160 mm Hg and/or diastolic blood pressure of ≥100 mm Hg, requiring two or more additional intensive medications than before) that were observed within three months after the initiation of Cfz therapy. The diagnosis of TMA was made as follows: when clinical findings were consistent with microangiopathic hemolytic anemia, such as thrombocytopenia, progressive anemia with schistocytes, elevated lactate dehydrogenase, and other differential diseases, including bacteremia or tumor progression, could be excluded.
Results
The patients' median age at Cfz initiation was 71 (range, 30-91) years. In total, 96 (100%), 86 (89.6%), 22 (22.9%), and 23 (23.9%) patients received bortezomib, lenalidomide, daratumumab, and autologous stem cell transplantation before Cfz therapy.
Until the end of the study, 13 CVAEs (13.5%) of grade ≥3 were observed, including 2 (11.1%), 1 (11.1%), and 10 (15.2%) in patients who received Kd (Cfz and dexamethasone), DKd (Daratumumab, Cfz, and dexamethasone), and KRd (Cfz, lenalidomide, and dexamethasone), respectively. Despite the use of appropriate supportive care, grade ≥3 AEs resulting in Cfz withdrawal were noted in 25 patients: CVAEs in 11, thrombocytopenia in 4, TMA in 3, and infection in two patients. Notably, one patient with end-stage myeloma who received KRd 20/27 mg/m 2 died of CVAE on day 10 of cycle 1.
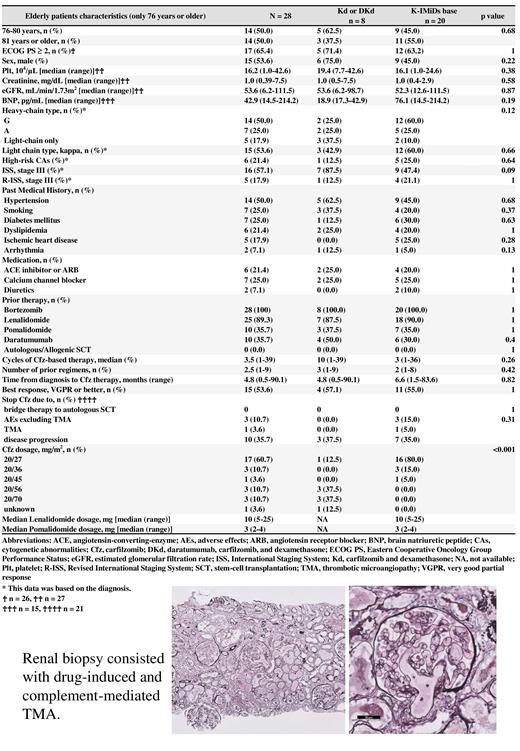
At Cfz initiation, 28 patients were aged 76-80 (n=14) and ≥81 years (n=14) (Fig. 1). Three elderly patients had severe CVAEs (10.7%). The median numbers of prior chemotherapy to Cfz, time to next treatment, and overall survival from Cfz initiation were 2.5, 28.0, and 41.1 months, respectively. Although 10 patients (35.7%) discontinued Cfz therapy due to disease progression, only four patients (14.3%) discontinued Cfz owing to grade ≥3 AEs, including TMA (n = 1) and CVAEs (n = 3).
Overall, five patients (5.2%) showed TMA. All patients received Kd-based regimens upon TMA development (Kd, n = 2 and DKd, n = 3). The median age of the patients who developed TMA was 74 years, and the Cfz dose was 20/27 in 1, 20/36 in 1, and 20/56 mg/m 2 in three patients. Although three patients developed TMA during the first Cfz cycle (on days 11, 16, and 21, respectively), two patients received KRd therapy before the Kd therapy that caused TMA. Two patients required temporary dialysis and four patients discontinued Cfz therapy. Eculizumab was administered in a patient and a kidney biopsy showed that the lumen of most glomerular capillaries was occluded by fibrin thrombi (Fig. 1). Furthermore, the vessel in the vascular pole of the glomerulus had necrotic lesions, suggesting drug-induced and complement-mediated TMA.
Conclusion
Cfz could be used safely in the elderly patients. The incidence of CVAEs by Cfz in elderly patients was similar to that in the entire cohort. The incidence of TMA was 5.2%, and Cfz-induced TMA should be carefully considered, particularly in patients using high-dose Cfz.
No relevant conflicts of interest to declare.