Abstract
Introduction: Altered cellular metabolism is a hallmark of every cancer cell. Aerobic glycolysis ("The Warburg Effect") is one of the earliest recognized metabolic abnormalities in cancer cells whereby extracellular glucose is preferentially metabolized and eventually processed to generate lactate and energy in the form of ATP before the former is released extracellularly, irrespective of oxygen availability. While extracellular lactate produced and released from cancer cells has traditionally been considered a waste metabolic by-product, recent understanding of cell metabolism suggests that it can also serve as a primary metabolic fuel for cancer cells via uptake by monocarboxylate transporters (MCTs). Our goal was to evaluate this "Reverse Warburg Effect" phenomenon in multiple myeloma (MM) cells and determine if it can be exploited for therapeutic purposes.
Methods: All HMCLs, MM1S, RPMI-8226 and U266, were grown in RPMI-1640 cell culture medium containing 11 mM glucose and supplemented with 10% dialyzed fetal bovine serum (FBS) and 2 mM Glutamine. Primary MM cells were extracted using magnetic bead CD138 positive selection from MM patient bone marrow aspirates. For 13C-labeling experiments, HMCLs and primary MM cells were suspended in RPMI-1640 cell culture media containing 13C-labeled isotopes. Isotopomer analysis of glycolytic and tricarboxylic acid (TCA) cycle metabolites from HMCL and primary MM cell pellets was performed using Agilent Technologies 5975C gas chromatography-mass spectrometry. Small molecule inhibitors, AZD3965 and syrosingopine, were purchased from Selleck Chemicals and Sigma respectively. Cellular viability and proliferation were measured using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrasodium bromide (MTT) and CCK-8 assays respectively. MCT-1 and MCT-4 antibodies for western blotting were utilized to evaluate their cell membrane expression on HMCLs.
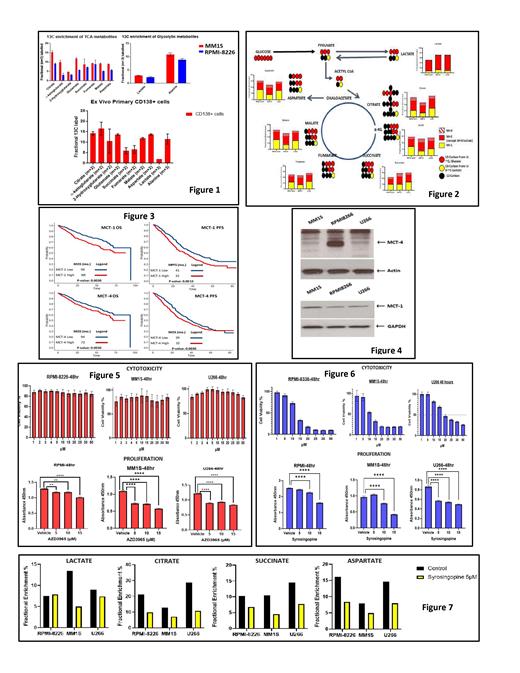
Results: The HMCLs, MM1S and RPMI-8226 as well as primary CD138+ cells from MM patient bone marrow were cultured in cell culture media containing physiological levels (1 mM) of U-13C-Lactate. The incorporation of extracellular 13C into the intracellular glycolytic and TCA cycle metabolite pool was observed (Fig 1) based on the expected isotopomeric patterns, demonstrating the Reverse Warburg Effect in MM cells. The relative contribution of carbon substrate by extracellular lactate compared to extracellular glucose was assessed in the following HMCLs: MM1S, RPMI-8226 and U266 cells by culturing in cell culture media containing 3-13C-Lactate and U-13C-Glucose. Extracellular lactate (yellow bar) contribution to the formation of TCA metabolites equaled that of glucose (red bar) based on the expected isotopomer patterns, suggesting the relative importance of extracellular lactate as an essential nutrient like glucose (Fig 2). Since MCT-1 and MCT-4 are key bidirectional cell membrane transporters of lactate in and out of cells, we explored the clinical significance of their gene expression level on clinical outcomes using the COMMPASS dataset provided by the Multiple Myeloma Research Foundation (MMRF). When MM patients were dichotomized at above or below the median of the expression levels of fragments per kilobase of transcript per million (FPKM), MCT-1 and MCT-4 overexpression conferred a worse progression free survival and overall survival (Fig 3). The MCT-1/MCT-4 protein expression was detectable across the various HMCLs: MM1S, U266 and RPMI-8226 (Fig 4). Inhibition of MCT-1 by specific inhibitor AZD3965 was able to reduce proliferation but not affect viability of HMCLs at 48 hours (Fig 5). However, dual inhibition of MCT-1/MCT-4 using syrosingopine was able to significantly reduce proliferation and decrease viability of HMCLs in a dose dependent fashion (Fig 6). Finally, dual inhibition of MCT-1/MCT-4 using syrosingopine reduced the utilization of extracellular lactate into the TCA cycle pool by HMCLs in media containing 3-13C-Lactate (Fig 7).
Conclusion: Utilization of extracellular lactate via Reverse Warburg Effect phenomenon appears highly active in MM cells. Disrupting the utilization of extracellular lactate by dual inhibition of both MCT-1 and MCT-4 appears therapeutic. In the future, dual inhibition of MCT-1/MCT-4 in combination with other anti-MM therapies should be evaluated to determine synergistic therapeutic potential.
Kumar: Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Carsgen: Research Funding; Sanofi: Research Funding; Novartis: Research Funding; Antengene: Consultancy, Honoraria; Beigene: Consultancy; Bluebird Bio: Consultancy; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tenebio: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Roche-Genentech: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding.