Abstract
Background: The Revised International Prognostic Scoring System (IPSS-R) classifies pts with MDS into risk categories, from very low-risk (vLR) to very high-risk (vHR), which guide treatment (tx) options. Pts with intermediate-risk MDS (IR-MDS) are heterogenous and real-world tx practices and outcomes for these pts are unknown. In Spain, the GESMD is an MDS registry containing more than 16,000 pts registered from 142 study sites which represent the country. We evaluated the real-world tx patterns and survival outcomes of pts with MDS from this registry across IPSS-R risk groups (with a focus on IR-MDS) and explored factors driving tx decisions among pts with IR-MDS.
Methods: We analyzed the data collected by the GESMD registry from January 2008 to June 2020. Pts included were adults diagnosed with MDS, with informed consent and ≥6-mo follow up if the pt was alive. Pts who did not have available data on their MDS risk score or their use of hypomethylating agent for MDS were excluded. Prior to inclusion in the registry, a curation process was performed to ensure that each pt had the minimum data set and to avoid duplication and errors. Data queries were responded to by physicians at study sites, and the final data included were reviewed by the investigators. Descriptive statistics were used to summarize demographics, clinical, and tx characteristics overall and by risk groups. Overall survival (OS) was analyzed by risk group and tx type using the Kaplan-Meier estimator and 95% confidence intervals (CIs) were calculated. In pts with IR-MDS, a number of baseline variables (including sex, diagnosis year, transfusion status, risk score, age, and blood and blast counts) were explored to assess their relationship with tx selection between either azacitidine (AZA) or best supportive care (BSC) only (eg, transfusions, growth factors).
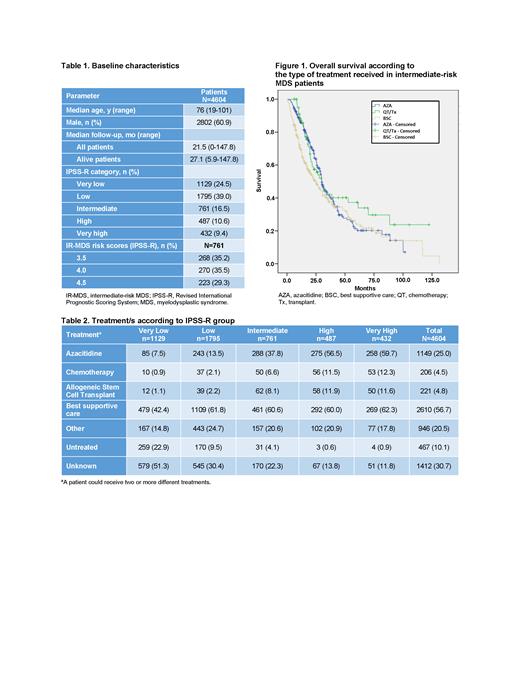
Results: In total, 4,604 pts were included in this analysis. The median age of enrolled pts was 76 y and 39% were female. Other baseline characteristics are seen in Table 1. Seven hundred sixty-one pts (16.5%) were classified as IR-MDS with similar distribution across IPSS-R risk score subgroups 3.5, 4.0, and 4.5. Txs received by pts in the cohort included AZA, chemotherapy, allogeneic stem cell transplant (alloSCT), BSC, and others (eg, immunosuppressors, androgens; Table 2). The majority of pts with IR-MDS were treated with BSC (61%) and AZA (38%). The median time from diagnosis to start of AZA tx ranged from 1 mo in pts with high-risk (HR) and vHR-MDS to 26 mo in pts with vLR-MDS; pts with IR-MDS started AZA at a median of 3 mo from diagnosis. Survival analysis by IPSS-R group shows that median OS decreased with increased risk score (vLR: 81.3 mo [95% CI, 73.6-89.0], low-risk (LR): 59.0 mo [95% CI, 55.2- 62.8], IR: 29.4 mo [95% CI, 27.0-31.8], HR: 15.2 mo [95% CI, 13.3-17.1], vHR: 9.4 mo [95% CI, 7.7-11.1]). When analyzed by tx type, pts with IR-MDS had longer median OS when treated with AZA or chemotherapy ± alloSCT than with BSC (AZA: 30.1 mo [95% CI, 27.3-32.9], chemotherapy ± alloSCT: 30.1 mo [95% CI, 23.8-36.4], BSC: 24.6 mo [95% CI, 18.5-30.7]; Figure 1). No relevant factors associated with decision to treat pts with IR-MDS with AZA or BSC were identified.
Conclusions: This study shows that more than one-third of pts with IR-MDS were treated with AZA shortly after their diagnosis, similar to the HR/vHR group. This cohort represents what we know about MDS and its distribution by risk group, while bringing new information on the use of AZA and its potential value in improving the OS of pts with IR-MDS. It is possible that other factors not included in the analysis (comorbidities, distance to the hospital, social and familial support) might have had an influence on the decision to treat pts with IR-MDS with AZA or offer BSC instead. Selection bias, misclassification, and confounding might occur when using registry data and this may limit the interpretation; however, the study shows important insights into the use of real-world therapies for pts with MDS, especially IR-MDS. The COVID-19 pandemic had an impact on the study, delaying the availability and curation process of data from recent years. Analysis of length of AZA tx in relation to response to tx is ongoing.
Diez-Campelo: BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Sasse: Novartis: Current Employment, Current holder of stock options in a privately-held company. Wormser: Roche: Current equity holder in publicly-traded company; Novartis: Current Employment, Current equity holder in publicly-traded company. Hernandez Donoso: Novartis: Current Employment, Current holder of stock options in a privately-held company. Colicino: Novartis: Current Employment, Current holder of stock options in a privately-held company. Tormo: Astellas, Novartis, Jazz, Pfizer, Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sanz: Gilead Sciences: Other: Travel, accommodations, and expenses; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Boehringer Ingelheim: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Helsinn Healthcare: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses, Research Funding. Díaz-Beyá: Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Jerez: Novartis: Consultancy; BMS: Consultancy; GILEAD: Research Funding. Valcárcel: Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizzer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jansen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene/BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Sabatolimab is a novel immuno-myeloid therapy targeting TIM-3 and is under investigation for the treatment of patients with myeloid malignancies