Abstract
Background: There is an intimate link between inflammation and thrombosis, and patients with pro-inflammatory/infectious disorders develop a hypercoagulable state. Extant coagulation assays are unable to distinguish the pro-coagulant state of a patient's blood, require 2-3 mL of blood, and take 2-3 hours for processing. These assays are also typically examined in plasma and do not represent the contribution of blood cellular elements that participate in thrombosis in vivo. Thus, a point-of-care device for rapid, comprehensive assessment of whole blood coagulation is crucial to ensure appropriate and timely evaluation in critically ill patients. We have introduced a microfluidic sensor (ClotChip) that uses dielectric spectroscopy to provide such an assessment in a handheld platform. We have shown in clinical studies in patients with a hypocoagulable state that ClotChip is sensitive to both coagulation factor and platelet defects, allowing for a global assessment of blood coagulation status using <10 µL of whole blood and in <30 min. In this study, we optimized ClotChip to assess the blood coagulation status in patients with a hypercoagulable state.
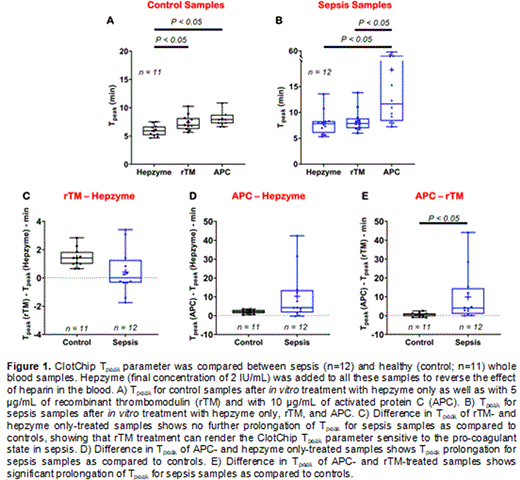
Methods: Citrated blood samples from 12 patients with a diagnosis of sepsis and 11 healthy donors as controls were obtained under an IRB-approved protocol and tested with ClotChip within 2 hours of collection. ClotChip readout curve was calculated as the temporal variation of blood dielectric permittivity at 1 MHz, and the time to reach a permittivity peak (T peak) was taken as an indicator of coagulation time based on our prior studies. To increase the sensitivity of the ClotChip T peak parameter to a hypercoagulable state, we used two different anticoagulants, recombinant thrombomodulin (rTM) and activated protein C (APC). To optimize the anticoagulant concentration, whole blood samples from healthy donors were treated in vitro with lipopolysaccharide to mimic a pro-coagulant state of blood and tested with ClotChip after adding various concentrations of rTM and APC. We concluded that a concentration of 5 µg/mL for rTM and 10 µg/mL for APC would result in an optimal change in T peak for detecting the pro-coagulant state. Since heparin (or lovenox) is routinely used in hospitalized patients, sepsis and control samples were pretreated with hepzyme at a final concentration of 2 IU/mL to reverse the heparin effect. The T peak parameter was measured and compared in (i) hepzyme only-, (ii) rTM-, and (iii) APC-treated samples. Data are reported as mean ± standard deviation. Two-tailed t test is used to test for statistical significance between groups, and P < 0.05 is considered statistically significant. In box-and-whiskers plots, the box represents the range from the first to the third quartile, the horizontal line represents the median, plus sign (+) represents mean of the data; whiskers extend to the maximum and minimum data values, and dots represent individual subject data.
Results: In hepzyme only-treated samples, T peak was significantly prolonged at 478±137 sec in sepsis samples, as compared to 357±58 sec in controls (Figs. 1A, 1B). rTM treatment resulted in T peak of 503±128 sec for sepsis samples and 443±81 sec for controls, whereas APC treatment resulted in T peak of 1,095±850 sec for sepsis samples and 477±71 sec for controls (Figs. 1A, 1B). Although T peak was prolonged at baseline in hepzyme only-treated sepsis samples, no further prolongation was noted with rTM treatment (difference in T peak of 24±94 sec; Fig. 1C), as compared to rTM-treated controls (difference in T peak of 85±40 sec; Fig. 1C). However, with a difference in T peak of 616±804 sec, the APC-treated sepsis samples exhibited T peak prolongation when compared to hepzyme only-treated sepsis samples, whereas the APC-treated controls did not (difference in T peak of 119±64 sec; Fig. 1D). A comparison between the APC- and rTM-treated samples revealed a significant prolongation of T peak in sepsis samples (difference in T peak of 591±815 sec) when compared to controls (difference in T peak of 30±66 sec; Fig. 1E).
Conclusions: Our studies identify a unique coagulation profile in sepsis patient blood using a microfluidic dielectric sensor. These data suggest that the addition of rTM or APC can enhance the sensitivity of the ClotChip T peak parameter for detecting the pro-coagulant state in whole blood. Ongoing studies are examining the coagulation profile in other pro-inflammatory and infectious states.
Suster: XaTek Inc.: Consultancy, Current holder of stock options in a privately-held company, Patents & Royalties, Research Funding. Mohseni: XaTek Inc.: Consultancy, Current holder of stock options in a privately-held company, Patents & Royalties, Research Funding. Nayak: BioChip Labs: Current Employment.