Abstract
Introduction: CAR T-cells have demonstrated remarkable ability to induce complete remission in patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r ALL). This success, however, is tempered by the toxicities associated with CAR T-cell therapy. Much has been published on cytokine release syndrome (CRS), but, to date, a comprehensive profile of specific end organ toxicities secondary to CAR T-cell therapy in the pediatric and young adult population is lacking.
Methods: This retrospective, single center study, was performed to characterize the specific adverse events (AEs) experienced by pediatric and young adult patients during the first 30 days following CAR T-cell infusion. AEs graded using Common Terminology Criteria for Adverse Events (CTCAE) were collected from all patients with r/r ALL treated on one of three phase I CAR T-cell trials (CD19, CD22, and CD1922) at the Pediatric Oncology Branch of the National Cancer Institute at the National Institutes of Health from 2012-2020. The primary objective was to determine the incidence of all severe AEs, defined as > grade (Gr) 3 AEs, overall and by organ system, attributed to research or disease. Secondary objectives were to stratify severe AEs based on development of CRS and CRS grade (using ASTCT CRS grading criteria). Descriptive statistics were reported along with comparisons of continuous parameters using Mann-Whitney and binomial parameters using Fischer's exact tests.
Results: We reviewed AE data from 134 patients with r/r ALL receiving one of 3 unique CAR T-cell constructs (Table). The median age was 15.2 years (Interquartile range (IQR) 9.5-21.2). The median number of prior therapies was 5 (IQR 3-6) and 57% had received a prior hematopoietic stem cell transplant (HSCT).
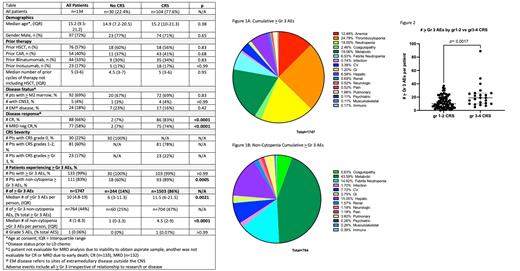
Amongst the 134 patients, a total of 1747 individual > Gr 3 AEs were experienced by 133 patients (99%) during the first 30 days following CAR infusion (Figure 1A). The median number of > Gr 3 AEs per patient was 10 (IQR 4.8-19).
Cytopenias (including neutropenia, thrombocytopenia and anemia) comprised the vast majority of total > Gr 3 AEs (n=983, 56.3%). The most common severe (> Gr 3) AEs were thrombocytopenia (n=433, 24.8%), metabolic abnormalities (i.e. electrolyte derangements) (n=333, 19.1%), neutropenia (n=332, 19%), and anemia (n=218, 12.5%).
With exclusion of cytopenias, 764 > Gr 3 AEs were experienced by 111 patients (83%), with a median of 4 (IQR 1-8.3) > Gr 3 AEs per patient. One grade 5 pulmonary AE occurred in the setting of acute respiratory distress syndrome (ARDS). Focusing on non-cytopenia AEs (Figure 1B), metabolic AEs made up 43.6% of AEs; hepatic toxicities (n=115, 15%), febrile neutropenia (n=114, 14.9%), and cardiovascular toxicities (n=59, 7.7%) were the next most frequent.
Of the 134 patients, 104 (77.6%) developed CRS. All 30 patients without CRS had at least 1 > Gr 3 AE (median 6, IQR 3-11.3). In contrast, the median number of > Gr 3 AEs in those with CRS was 11.5 (IQR, 6-21.5), (p=0.0021). When stratified by CRS Gr 1-2 versus CRS Gr 3-4 (Figure 2), patients with higher-grade CRS also had a higher median number of > Gr 3 AEs per patient (p= 0.0017).
Conclusions: Among 134 children and young adults with r/r ALL receiving phase I CAR T-cells, we found a high incidence (99%) of severe AEs, with a per patient median of 10 (IQR 4.8-19) > Gr 3 AEs. While the majority of > Gr 3 AEs were cytopenias, 17 different categories of AEs were experienced. The development and severity of CRS associated with an increase in the median number of severe AEs per patient. As phase I trials of CAR T-cell therapy expand, it is imperative to understand the full toxicity profile of these therapies. While the definition and refined grading of CRS has helped advance the field, there is a gap in knowledge regarding patient specific end-organ toxicities beyond CRS. Our data help establish a foundation for the full toxicity profile experienced by patients enrolling on phase I CAR T-cell trials. With an emerging role for earlier intervention for CRS, we anticipate that the toxicity burden will decrease. Next steps include characterizing the specific toxicities within each AE category, evaluating duration and time to resolution, distinguishing attribution to research versus disease and studying the impact of earlier use of tocilizumab on toxicity profile. Future directions will incorporate assessment of baseline organ function pre-CAR and its impact on development of post CAR severe AEs.
No relevant conflicts of interest to declare.