Abstract
Chimeric antigen receptor-engineered T cells targeting CD19 (CART19) have revolutionized the management of relapsed and refractory B cell malignancies. Despite high initial response rates, many patients with acute lymphoblastic leukemia (ALL) ultimately relapse after CART19. In contrast, most patients with non-Hodgkin lymphoma experience only partial or no responses. Collectively, <50% of patients treated with CART19 achieve durable disease remission. Identification of the biology responsible for these failures is central to improving CAR T cell efficacy. Several clinical reports have demonstrated that a common cause of resistance to CART19 is antigen escape, in which ALL clones emerge that have lost surface expression of CD19. The mechanisms of antigen escape that have been recognized to date all rely on disruption of CD19 genomic loci or transcribed CD19 mRNA; alterations of fully-translated CD19 protein that lead to CART19 failure have not been described.
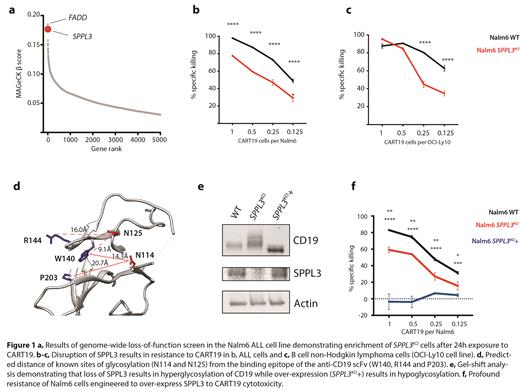
To identify pathways responsible for enabling tumor-intrinsic resistance to CART19 we performed a genome-wide loss-of-function screen in the Nalm6 ALL cell line. The second-most enriched gene in this screen was SPPL3 (Figure 1a), encoding a Golgi-resident aspartyl protease. Previous studies have determined that SPPL3 functions to broadly limit protein glycosylation by cleaving glycosyltransferases from the Golgi membrane, impairing their ability to add complex glycans to proteins as they pass through the Golgi (Voss M. et al. EMBO, 2014). Using targeted genomic disruption, we confirmed that loss of SPPL3 results in resistance to CART19 in human ALL and non-Hodgkin lymphoma models (Figures 1b-c). CART19 cells exposed to SPPL3KO ALL demonstrated significantly lower expression of CD69, PD1, Tim3 and CD107a, as well as less activation of the central T cell transcription factors NFAT and NFκB, indicating a global suppression of T cell stimulation.
Consistent with its known function, loss of SPPL3 resulted in increased addition of complex glycans to CD19. Surface staining of SPPL3KO cells revealed that CD19 antibodies were less capable of binding this hyperglycosylated CD19. This included decreased binding of the antibody used to construct the anti-CD19 CAR (clone FMC63). Protein modeling revealed that an asparagine residue known to be normally glycosylated on CD19 (N125) is in close physical proximity to the FMC63 binding site (Figure 1d), suggesting that the addition of complex glycans at this site may be responsible for disruption of CAR binding that led to impaired T cell activation. We next turned our attention to CD22, another B cell antigen that is normally glycosylated and the target of CAR therapy. In contrast to CD19, loss of SPPL3 had no impact on CD22 glycosylation or antibody binding. Similarly, loss of SPPL3 did not enable resistance to CD22-targeted CAR T cells. These findings substantiated our hypothesis loss of SPPL3 lead to CART19 failure directly via modifying CD19 glycosylation, and not through another CD19-independent mechanism. To further validate the impact of CD19 glycosylation in regulating CART19 efficacy, we over-expressed SPPL3 in ALL cells, previously shown to promote global hypoglycosylation. We confirmed decreased glycosylation of CD19 (Figure 1e), and found that this resulted in loss of FMC63 binding to CD19 and complete resistance to CART19 activity (Figure 1f).
In summary, our findings identify that changes to CD19 glycosylation, either enhanced or decreased, impair the ability of CARs to bind and initiate T cell effector function against malignant B cells. Further, these data identify post-translational protein modification as a novel mechanism of antigen escape from CAR-based T cell immunotherapy.
Ruella: AbClon: Consultancy, Research Funding; viTToria biotherapeutics: Research Funding; BMS, BAYER, GSK: Consultancy; Novartis: Patents & Royalties; Tmunity: Patents & Royalties. Gill: Interius Biotherapeutics: Current holder of stock options in a privately-held company, Research Funding; Novartis: Other: licensed intellectual property, Research Funding; Carisma Therapeutics: Current holder of stock options in a privately-held company, Research Funding.