Abstract
Purpose
The improved survival for mantle cell lymphoma (MCL) patients calls for updated knowledge about long-term adverse effects of treatment. We recently showed that younger MCL patients (≤65 years of age) selected for rituximab-cytarabine containing regimens and a consolidating high-dose chemotherapy with stem cell transplantation (HD-ASCT) had a 42% reduced all-cause mortality compared to patients selected for less intensive regimens with rituximab-bendamustine and/or rituximab-CHOP based treatment without HD-ASCT (non-HD-ASCT) (Blood Advances 2021, PMID: 3371034). The aim here was to describe long-term side-effects and outcome from these different treatment strategies.
Methods
We conducted a population-based cohort study including all Swedish MCL patients diagnosed between 2000 and 2014, and 1:10 matched comparators. First, transplantation-related mortality and acute side effects (within 60 days of HD-ASCT) were described. Next, for the analysis of late effects, we took a landmark approach with follow-up starting at 12 months after diagnosis (i.e., assuming treatment completion). Hazard ratios with 95% confidence intervals (CIs) of first hospital visit/admission/death due to a specific disease group (based on ICD chapters) were estimated using Cox regression models, comparing the rates among HD-ASCT and non-HD-ASCT patients to those among the comparators. Additionally, the 5-year cumulative incidence of death due to MCL, other malignancy, cardiovascular disease, or remaining causes was described for both patient treatment groups (HD-ASCT and non-HD-ASCT) and comparators.
Results
A total of 620 MCL patients were included with a median follow-up from diagnosis of 4.1 years (range: 0-15.7). In total, 280 (45%) received HD-ASCT (median age 58, range 32-69) and 340 (55%) R-CHOP/R-Bendamustin and non-HD-ASCT (median age 65, range 22-69). Four out of 280 (1.5%) HD-ASCT treated patients died within 60 days of their transplantation. Acute side effects of transplantation were mainly infections and diseases of the circulatory or digestive systems.
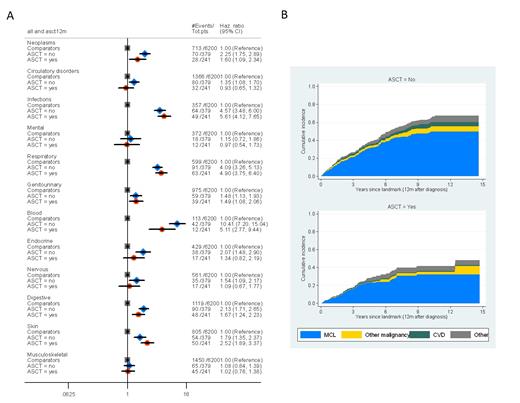
In relation to the 6,200 matched comparators (median age 63, range 22-70) the rate of hospital visits/admissions/death after 12 months was significantly higher among MCL patients (irrespective of treatment intensity) for neoplasms (excluding MCL), other blood disorders, infections, respiratory, genitourinary, digestive, and skin disorders (Figure 1A). Patients treated with HD-ASCT did not experience a higher rate of late effects than those treated without HD-ASCT, for any of the disease groups (Figure 1A).
The 5-year cumulative incidence of MCL-specific death in HD-ASCT treated patients was 0.23 (95% CI: 0.18-0.30) and 0.32 (95% CI: 0.26-0.38) in non-HD-ASCT treated patients (Figure 1B). Fatal long-term (≥12 months) side effects were rare in HD-ASCT treated patients, and the absolute majority of MCL patients died from their lymphoma and not from a complication.
Conclusions
MCL patients have a higher risk of long-term complications than comparators particularly in the disease groups respiratory disorder, blood disorders (other than MCL) and infections. Intensive first-line treatment including HD-ASCT was however associated with a similar rate of long-term side-effects in all disease groups as among patients selected for less intensive treatments and non-HD-ASCT. Hence, given the previously shown favourable long-term survival associated with HD-ASCT, it appears that this treatment should not be avoided based on fear of neither fatal acute side effects nor more long-term side effects. Instead symptomatic lymphoma disease and death from the lymphoma seem to drive the majority of long-term health problems in MCL patients regardless of treatment intensity.
Figure 1. A) Hazard ratios for hospital visits/admissions/deaths due to specific disease groups (based on ICD chapters) among MCL patients and comparators, stratified by treatment with consolidative autologous stem cell transplant (ASCT) or (non-ASCT). All models were adjusted for age at diagnosis, sex, calendar year of diagnosis, and Charlson comorbidity index. Grey dots; comparators, blue dots MCL patients (non-ASCT-treated) and red dots MCL patients (ASCT treated) B) Cumulative incidence of death due to MCL, other malignancies, cardiovascular disease (CVD), and other causes among MCL patients without (upper panel) and with (lower panel) ASCT.
Ekberg: Jansen-Cilag: Other: part of a research collaboration between Karolinska Institutet and Janssen Pharmaceutica NV for which Karolinska Institutet has received grant support. Smedby: Jansen-Cilag: Other: part of a research collaboration between Karolinska Institutet and Janssen Pharmaceutica NV for which Karolinska Institutet has received grant support. Albertsson-Lindblad: Jansen-Cilag: Other: part of a research collaboration between Karolinska Institutet and Janssen Pharmaceutica NV for which Karolinska Institutet has received grant support. Jerkeman: Jansen-Cilag, AbbVie, Gilead, Celgene: Other: part of a research collaboration between Karolinska Institutet and Janssen Pharmaceutica NV for which Karolinska Institutet has received grant support, Research Funding. Weibull: Jansen-Cilag: Other: part of a research collaboration between Karolinska Institutet and Janssen Pharmaceutica NV for which Karolinska Institutet has received grant support. Glimelius: Jansen-Cilag: Other: part of a research collaboration between Karolinska Institutet and Janssen Pharmaceutica NV for which Karolinska Institutet has received grant support.