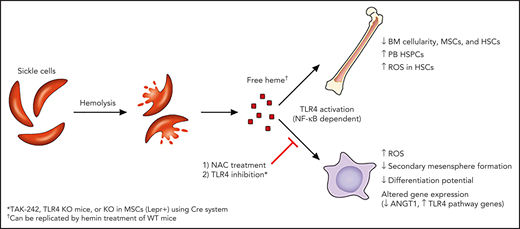
In this issue of Blood, Tang et al,1 using sickle cell disease (SCD; Townes transgenic) mice, report defects in bone marrow (BM) mesenchymal stromal cells (MSCs) and hematopoietic stem cells (HSCs) related to chronic heme exposure, which can be reversed through treatment with N-acetylcysteine (NAC) or inhibition with Toll-like receptor 4 (TLR4).
MSCs are known to be important in supporting the BM stroma, including HSCs.2 Tang et al provide an in-depth evaluation of murine SCD MSCs, demonstrating many defects in the SCD BM space. Although the pathophysiology is certainly multifactorial, these results may help explain the difficulty in mobilizing autologous HSCs in patients3 with SCD and higher rates of graft rejection after allogeneic hematopoietic cell transplantation (HCT).4,5
Tang et al hypothesized that free heme (released via hemolyzed SCD red blood cells [RBCs]) induces oxidative stress, altering the SCD BM microenvironment, most notably MSCs (see figure). Previous work examining human SCD MSCs (median age, 8.3 years) demonstrated comparable basic phenotype and function of MSCs, compared with those of healthy controls.6 They performed a more detailed in vitro and in vivo evaluation of murine SCD MSCs. They found defects in MSCs from Townes mice, including decreased frequency in the BM, increased reactive oxygen species (ROS), decreased ability to form secondary mesenspheres, and decreased adipogenic and osteogenic differentiation potential. Decreased BM HSCs, increased HSC ROS, and increased peripheral blood (PB) hematopoietic stem and progenitor cells (HSPCs) were also documented. Further, expression of key genes involved in HSC maintenance was downregulated in SCD MSCs, with upregulation of TLR4 and related genes. Notably, preceding coculture with SCD MSCs (compared with wild-type [WT]) led to decreased engraftment of WT HSCs, with increased HSC ROS found 16 weeks after transplant. Many of these findings were confirmed in WT mice treated with hemin, implicating free heme in the induction of these downstream effects.
Free heme induces defects in SCD murine MSCs and HSCs in a TLR4-dependent manner. Hemolysis of sickle RBCs leads to release of free heme, which drives changes in BM MSCs and HSCs, including upregulation of ROS. These defects can be reversed in vivo or in vitro by targeting oxidative stress (NAC) or TLR4 pathways. ANGT1, angiopoietin 1. Professional illustration by Patrick Lane, ScEYEnce Studios.
Free heme induces defects in SCD murine MSCs and HSCs in a TLR4-dependent manner. Hemolysis of sickle RBCs leads to release of free heme, which drives changes in BM MSCs and HSCs, including upregulation of ROS. These defects can be reversed in vivo or in vitro by targeting oxidative stress (NAC) or TLR4 pathways. ANGT1, angiopoietin 1. Professional illustration by Patrick Lane, ScEYEnce Studios.
Most important, the defects in MSCs and HSCs exposed to free heme were reversible. First, treatment with NAC, in vitro or in vivo, reversed these findings, implicating oxidative stress downstream of exposure to free heme. Second, TLR4 inhibition reversed findings in hemin-treated (WT mice receiving the TLR4 inhibitor TAK-242 or TLR4 knockout [KO] mice) and SCD mice (TAK-242 treated). This effect was specific to MSCs, as depletion of TLR4 in only Lepr+ cells (MSCs) led to decreased PB HSPCs. Further experiments implicated NF-κB downstream of TLR4, with downregulation of p65 in MSCs from TAK-242–treated SCD mice. Last, transfusion of healthy RBCs reversed these defects in SCD mice, most likely through increased hemopexin (which scavenges plasma heme). In preliminary studies extending their murine findings into patients with SCD, Tang et al found decreased ROS in PB multipotent progenitor cells only 4 hours after RBC transfusion.
Although Tang et al primarily used murine models, they set the stage for future preclinical evaluations of MSCs and HSCs from patients with SCD. Published clinical data support their hypothesis that such defects are found in patients with SCD. First, MSCs continue to be of recipient origin after allogeneic HCT.7 Thus, it is plausible that defective MSCs contribute to the higher risk of graft rejection seen in patients with SCD,5 including matched sibling donor HCT when myeloablative conditioning is used (before addition of antithymocyte globulin).4 Second, recent data from patients who undergo autologous HSC collection before gene therapy demonstrate BM CD34+ HSC yield lower than expected (compared with healthy controls), with lower CD34bright cells (a marker for long-term repopulating HSCs).3 With a move to plerixafor-mobilized PB CD34+ collection, CD34+ and CD34bright collection yields normalized to those in healthy control subjects,3 with some early data suggesting escalation of the plerixafor dose may further increase yield.8 Consistent with a disease-related defect, age >30 years, increased hospitalization frequency, and chronic pain were significantly associated with lower CD34+ HSC yield.9 If similar BM and MSC defects are confirmed in persons with SCD, these findings set the stage for potential clinical strategies to target these defects, including inhibition of TLR4 or oxidative stress pathways.
Conflict-of-interest disclosure: E.S. has served as an elected physician member of the board of directors of the International Society for Cell and Gene Therapy (ISCT) and as an elected member of the Scientific Executive Committee for the Sickle Cell Transplant Advocacy and Research Alliance (STAR), which has received research funding from bluebird bio, Inc.