In this issue of Blood, Lowe et al present results of a phase 2 study combining brentuximab vedotin (BV) with standard chemotherapy in the treatment of newly diagnosed pediatric anaplastic lymphoma kinase–positive (ALK+) anaplastic large-cell lymphoma (ALCL) and observe that it eliminated almost all relapses while receiving therapy, a significant breakthrough in the management of this disease.1
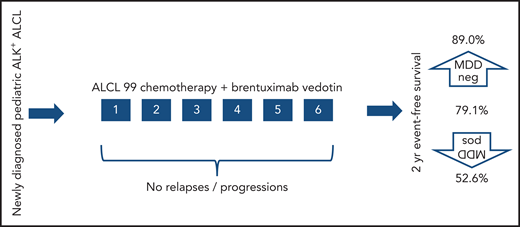
One-in-4 to 1-in-3 children treated for ALK+ ALCL will relapse, no matter what combination of conventional chemotherapy is used.2 There are several targeted therapies including ALK inhibitors and BV3,4 that have proven effective in the treatment of relapsed and refractory disease, but to date, none has been incorporated into frontline therapy. Lowe et al report on a study designed to investigate the safely and efficacy of one of these: BV3 combined with the internationally used standard chemotherapy regimen ALCL995 in children with newly diagnosed ALK+ ALCL. They show that BV can be safely combined with existing standard chemotherapy, which is significant because targeted agents do not always combine with conventional chemotherapy without unexpected toxicity emerging.6 In addition, for the first time, they report a trial of first-line therapy in this disease in which no relapses occurred during treatment (there was 1 relapse shortly after completion of therapy before reassessment, a rate of 1.5%), with previous studies having 4% to 8% of patients progress/relapse during therapy.5,7 The elimination of relapse or progression during therapy is a very significant goal, because it carries the poorest outcome in this disease.8
After a prephase, patients with newly diagnosed ALK+ ALCL received 6 cycles of chemotherapy with BV. No relapses before the completion of planned therapy were observed. One occurred after completion, but before final disease reassessment. Survival was favorable and the prognostic significance of MDD was confirmed.
After a prephase, patients with newly diagnosed ALK+ ALCL received 6 cycles of chemotherapy with BV. No relapses before the completion of planned therapy were observed. One occurred after completion, but before final disease reassessment. Survival was favorable and the prognostic significance of MDD was confirmed.
Lowe et al used a randomized noncomparative design to investigate the safety and efficacy of BV or crizotinib combined with standard chemotherapy . The primary outcomes were toxicity and event-free survival (EFS), with historical control used for evaluation of EFS. The study was not designed to compare BV with crizotinib; the results for the crizotinib arm will be reported independently. Children with a body surface area <0.9 m2 were nonrandomly assigned to the BV arm because of difficulties of administering the required dose of crizotinib due to the formulation available. Although smaller body surface area is associated with lower age, age is not of prognostic significance in pediatric ALCL,9 so this bias in the population should not influence the interpretation of the results.
BV is an anti-CD30 monoclonal antibody conjugated with the tubulin inhibitor monomethyl auristatin E, and in this study, it was given on the first day of each cycle of ALCL99 therapy. Lowe et al did not observe any increased toxicity compared with that expected from ALCL99, and in particular, there was none of the significant neurotoxicity that might be expected from a tubulin inhibitor. Furthermore, the EFS of 79.1% observed in this study compared favorably with the assumed 70% historical control used.
Several prognostic features have been reported that identify those children in whom frontline therapy is most likely to fail in ALK+ ALCL. Among those, the presence of minimal disseminated disease (MDD) detected in blood or bone marrow is the strongest.9 In their study, Lowe et al confirm that identification of MDD by reverse transcriptase polymerase chain reaction remains highly prognostic of treatment failure, even with the addition of BV. Those with no MDD detected had an EFS of 89%, whereas those with MDD detected had an EFS of 52.6% (see figure).
Much work remains to be done to establish the place in frontline therapy for a rising number of agents; however, Lowe et al have shown that BV has place in the treatment of patients with newly diagnosed disease and that relapse during therapy can be substantially overcome with its use. It remains to be seen how BV will be incorporated into future strategies that are less acutely toxic and more effective. The prospect of combining BV with other targeted therapy and reducing or eliminating conventional chemotherapy is one that can be explored based on the results of this study. Stumme et al have presented a single case report of the combination of crizotinib and BV combined in relapsed ALCL, with good efficacy and without limiting toxicity; however, this combination did not prevent central nervous system relapse,10 and so it seems likely that some form of conventional chemotherapy will continue to be needed, and further studies will be required to define the place of BV. For now, at least, there is additional, effective help on the frontline of therapy for children newly diagnosed with ALK+ ALCL.
Conflict-of-interest disclosure: G.A.A.B has received institutional consultancy fees from Roche, Takeda, Novartis, and Janssen.