Introduction: The past decade in AML research has led to increased emphasis on disease-related mutations and associated targeted therapies. FMS-like tyrosine kinase 3 (FLT3) mutations (FLT3+) are found in about 30% of AML patients and confer a poorer prognosis. Also, the availability of targeted therapies increases the value of testing for FLT3+ AML. A paucity of data exists regarding the real-world FLT3 testing rates in AML patients in both the newly diagnosed and R/R settings. As the treatment landscape for patients with R/R FLT3+ AML expands, it is important to understand how utilization of these therapies and trends in FLT3 testing impact patient outcomes in real-world settings. These updated results examined FLT3 testing trends, treatment patterns, and overall survival (OS) in patients with R/R FLT3+ AML. Patients were grouped based on the FDA approval of the second-generation FLT3 inhibitor gilteritinib (pre-gilteritinib approval vs. post-gilteritinib approval).
Methods: This ongoing (01/01/2015-4/17/2020) retrospective study uses electronic medical record data of US patients from a network of 400+ oncology practices maintained in the Definitive Oncology Dataset, including practices affiliated with CancerLinQ. Eligible adult (≥18 years) patients who had a confirmed diagnosis of AML, FLT3+ status, and had ≥1 R/R event between 01/01/2015 and 02/20/2020 were included. FLT3 testing trends included testing performed at initial diagnosis, re-testing performed in the R/R setting, and changes in FLT3 mutation status. Treatment patterns included all systemic anticancer therapies received (including supportive care) for R/R FLT3+ AML. OS was measured from the first R/R event; patients without evidence of death were censored at the last observed visit. Kaplan-Meier analysis was applied to evaluate differences in OS by subsequent hematopoietic stem cell transplant (HSCT) status.
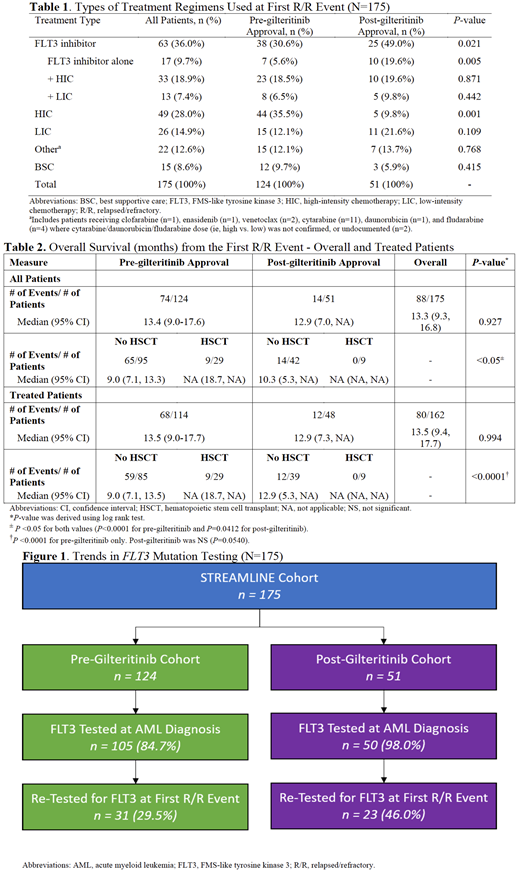
Results: Data from 175 patients (50.9% male, n=89) with R/R FLT3+ AML were evaluated (n=124 pre-gilteritinib; n=51 post-gilteritinib). Most patients were White (72.0%; n=126) with a median age of 62 years (range: 20-86) at first R/R event. Median length of follow-up was limited for the post-gilteritinib cohort (9.2 months vs. 15.0 months for pre-gilteritinib, P<0.001). Patients tested for FLT3 mutations at initial AML diagnosis increased from 84.7% (n=105/124) in the pre-gilteritinib cohort to 98.0% (n=50/51) in the post-gilteritinib cohort, and the rates of re-testing increased from 29.5% (n=31/105) to 46.0% (n=23/50) across the two cohorts (Figure 1). After the first R/R event, 18.9% (n=33/175) reported changes in their FLT3 mutation status. Seventy-four different treatment combinations were utilized at the first R/R event. Use of FLT3 tyrosine kinase inhibitors (TKIs), either alone or in combination with chemotherapy, increased from 30.6% (n=38/124) in the pre-gilteritinib cohort to 49.0% (n=25/51) in the post-gilteritinib cohort (Table 1). In the pre-gilteritinib cohort midostaurin (52.6%, n=20/38) and sorafenib (32.4%, n=13/38) were the most commonly prescribed FLT3 TKIs. In the post-gilteritinib cohort, the most common FLT3 TKI was gilteritinib (52.0%, n=13/25). Median OS (95% CI) across all patients at the first R/R event was 13.4 (9.0-17.6) months in the pre-gilteritinib cohort and 12.9 (7.0, NA) months in the post-gilteritinib cohort. Median OS was significantly shorter among patients that did not receive HSCT among all patients and the subset of treated patients (P<0.05 for both values). Median OS for patients with no HSCT was 3.9 months longer in the post-gilteritinib cohort versus the pre-gilteritinib cohort (Table 2).
Conclusion: An increase in FLT3 TKI use (both monotherapy and in combination) and a decrease in high-intensity chemotherapy was observed across the two cohorts. In the 18-month post approval period, FLT3 TKI use increased 60% with gilteritinib accounting for more than half of FLT3 TKIs used in the R/R setting. FLT3 re-testing increased by 55% in the R/R setting between the two cohorts; however, re-testing is suboptimal and there is a need to re-test as patients progress. Among pre- and post-treated patients that did not receive HSCT, there was an improvement in OS by almost 4 months, possibly due to increased use of targeted FLT3 TKIs. With recent approval of these targeted therapies, it will be important to continue to monitor FLT3 testing, treatment patterns, and clinical outcomes.
Zeidan:Celgene / BMS: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Otsuka: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; CCITLA: Other; Astex: Research Funding; Cardinal Health: Consultancy, Honoraria; Trovagene: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Leukemia and Lymphoma Society: Other; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Taiho: Consultancy, Honoraria; Aprea: Research Funding; ADC Therapeutics: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria; MedImmune/Astrazeneca: Research Funding; Acceleron: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Cardiff Oncology: Consultancy, Honoraria, Other. Gilligan:ConcertAI: Current Employment. Grinblatt:Astellas: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Alexion: Speakers Bureau. Elsouda:Astellas: Current Employment. Sullivan:Astellas Pharma: Current Employment. Pandya:Astellas Pharma, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.