Introduction: Multiple myeloma is an incurable hematologic malignancy. However, many novel drugs (lenalidomide, carfilzomib, daratumumab, elotuzumab and ixazomib) have recently been approved in Brazil for this disease. Proving that the availability of these drugs increases survival is not straightforward, since pivotal trials with these drugs were designed to show benefit in progression-free survival and not in overall survival.
Hypothesis: We have hypothesized that the availability of novel drugs would reduce the risk of death.
Methods: This is a real-world, unicenter analysis that included patients with multiple myeloma treated between 1993 and 2017. The availability of novel drugs (lenalidomide, carfilzomib, daratumumab, elotuzumab and ixazomib) was treated as a time-dependent covariate and analyzed by Cox model. The analysis was controlled for the International Staging System (ISS) and for age.
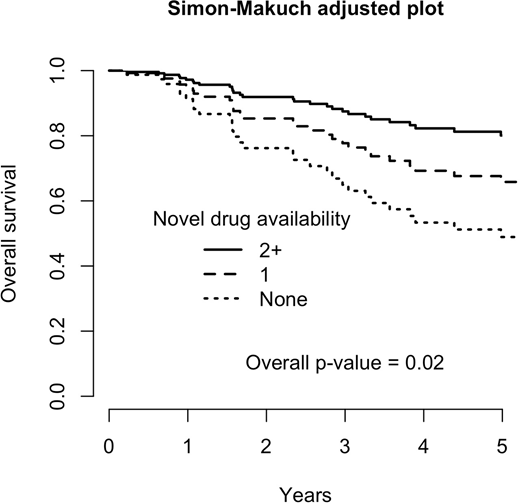
Results: With a median follow-up of 6 years, we included 151 patients with multiple myeloma. Fourty percent had ISS-I, 34% ISS-II and 26% ISS-III. Mean age was 60.5 y/o (SD: 12.2). There has been 51 deaths, and 5-y overall survival was 90% for ISS-I, 80% for ISS-II and 54% for ISS-III (p=0.0004). In multivariable analysis, age (HR=1.06, p<0.0001) and ISS-III (HR=4.51, p<0.0001) were risk factors for death, while the availability of two or more novel drugs was protective (HR=0.31, p=0.006). The availability of a single novel drug was also protective (HR=0.58), although it did not reach statistical significance (p=0.13). Adjusted Simon-Makuch survival plot is in figure 1 (adjusted for ISS III and 60 y/o).
Discussion:
Our results show that the availability of two or more novel drugs increases overall survival in multiple myeloma. This is an important finding, since the pivotal trials were designed to show advantages in progression-free survival and, once achieved, the experimental drug was offered to the control group, hampering overall survival analyses. Overall survival benefit, therefore, are more likely to be seen in real-world studies. We included the availability of novel drugs as a time-dependent, and the interpretation of our results is that patients alive when two novel drugs became available had benefit in overall survival. Our study has two weaknesses. Two or more novel drugs were more likely to be available for patients from a more recent time. This difference could be due to changes in supportive care, but there has been no major change in supportive care for multiple myeloma patients. Another weakness is that patient alive in the more recent years may have not received novel drug-based treatment. Considering that multiple myeloma is an incurable disease, this is unlikely for the majority of patients. In conclusion, the availability of two or more novel drugs for multiple myeloma improves survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.