Recent studies have identified several major qualitative and quantitative differences in important hemostatic proteins between adult and neonatal humans, including the primary coagulation protein fibrinogen.Despite these differences, neonates with post-operative bleeding from procedures requiring cardiopulmonary bypass (CPB) or extracorporeal membrane oxygenation (ECMO) are treated with transfusions of adult blood products, namely adult fibrinogen. The effectiveness of such transfusions is inconsistent in neonates and often results in a deficient fibrin matrix structure which may not be sufficient for mitigating bleeding. Our recent studies have also identified differences at the bulk clot level between purified neonatal and adult fibrin clots, including major structural and functional distinctions, which could contribute to these outcomes. Notably, adult fibrinogen degrades slower than neonatal clots, therefore transfusion of adult blood products to neonatal patients could contribute to thrombotic complications. Given the inconsistent results and potential complications from the transfusion of adult blood products to neonatal patients, there is a critical need to better understand the mechanistic differences in hemostatic processes between adults and neonates.
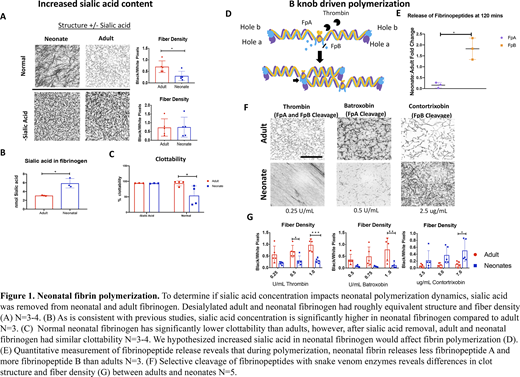
Neonates possess a molecular variant of fibrinogen known as fetal fibrinogen. Increased sialic acid concentration compared to adults has been identified in many neonatal glycoproteins across physiological systems, including fetal fibrinogen. Studies have shown that fibrin clot properties are influenced by fibrin polymerization mechanisms and post translational modifications (e.g. sialic acid). For example, an increased sialic acid content in the dysfibrinogenemia associated with liver disease has been associated with an altered fibrin clot structure. Additionally, recent work from our group has found that the increased sialic acid in neonatal fibrin networks results in significantly greater fibroblast attachment than adult networks. Therefore, we hypothesized that differences in neonatal and adult fibrin clot properties are due to mechanistic differences in fibrin polymerization between neonates and adults owing to altered sialic acid concentrations.
The activation of fibrinogen and conversion into fibrin by the proteolytic enzyme thrombin is essential for the formation of a stable blood clot and the cessation of bleeding. Thrombin converts soluble fibrinogen to insoluble fibrin via cleavage of fibrinopeptides A and B, exposing fibrin knobs A and B. Fibrin protofibrils are then formed from the noncovalent binding of fibrin knobs to complementary fibrin holes a and b on adjacent proteins. In adults, fibrin A:a knob:hole interactions are critical for polymerization. However, these mechanisms have not been explored in neonates. Therefore, we characterized the influence of sialic acid on the knob:hole interactions in neonatal fibrin polymerization.
We first investigated the influence of sialic acid concentration on neonatal fibrin polymerization by removing sialic acid via enzymatic digestion and performing structural and functional analysis on desialylated fibrinogen. Desialylated adult and neonatal fibrinogen had roughly equivalent structure, polymerization kinetics, and clottability results. These results indicate that differential sialylation may at least partially explain functional differences been adult and neonatal clots. Additionally, we investigated the role that sialylation plays on neonatal fibrin polymerization dynamics by comparing neonatal or adult fibrin clots formed with snake venom thrombin-like enzymes (svTLE) that preferentially cleave either A or B fibrinogen fibrinopeptides or thrombin. Structural, mechanical, fibrinolytic, and polymerization assays were conducted. Quantitative release of fibrinopeptides was determined via ELISA. Results indicate neonatal fibrin polymerization mechanisms are more dependent on B:b knob:hole interactions than adult fibrin. Results from this study provide insight into the mechanism of the neonatal clotting process and are a critical contribution for the development of neonatal-specific treatments for bleeding and thrombosis.
Brown:Selsym Biotech, Inc.: Other: Founder and CEO.
Author notes
Asterisk with author names denotes non-ASH members.