Introduction: Prognosis in patients (pts) with r/r DLBCL remains unsatisfactory. Pts at risk for treatment-failure cannot accurately be identified by current prognostic factors and are usually treated with standard salvage therapies. Therefore, identification of new prognostic factors in r/r DLBCL is essential for optimizing therapy with risk-adapted approaches. Metabolic tumor volume (MTV) is a strong independent prognostic factor in lymphoma, although, data in r/r DLBCL is limited. We explored the prognostic value of pre-salvage therapy PET/CT metrics in this population.
Methods: We performed a retrospective intent-to-treat analysis of pts with r/r DLBCL treated with platinum based-chemotherapy at Sylvester Comprehensive Cancer Center (6/2010 to 3/2020). Only pts having measurable disease on FDG PET/CT prior to the initiation of salvage chemotherapy were included. MTV was obtained by summing the metabolic volumes of all individual lesions using the 41% of SUVmax threshold. Total lesion glycolysis (TLG) was calculated as the product of MTV and SUVmean. The individual lesion or coalesced lesion with the maximum TLG was classified as TLGmax. The global TLG (sum of each individual lesion TLG) was evaluated as well. MTV, TLGmax, TLG global and SUVmax were calculated pre and post-salvage chemotherapy by a board-certified nuclear radiologist. Hermes Hybrid 3D software was implemented for these calculations. Statistical analysis methods included nonparametric Wilcoxon rank-sum test, logistic regression, and Cox's proportional hazards regression.
Results: A preliminary cohort of 79 pts was evaluated. Pts characteristics were as follows: median age 59 (range 20 to 82) years, Karnofsky performance status (KPS) ≥ 80: 71 (90%), elevated LDH: 42 (53.2%), stage III-IV: 64 (81%), and second-line age-adjusted IPI (sAAIPI) 0-1: 42 (53.2%) 2-3: 37 (46.8%). Distribution of salvage chemotherapy regimens was R-ICE 55 (70%), R-DHAP 13 (16%), R-Gem-Ox 10 (13%), and R-ESHAP: 1 (1%) pts. Treatment response was CR 30 (38%), PR 29 (36.7%) and progression of disease in 20 (25.3%) pts. After salvage chemotherapy 60 (76%) pts proceeded with cellular therapy [stem cell transplant (SCT): 41 (52%), CAR-T: 12 (15%), and SCT and CAR-T: 7 (9%) pts].
Using the 41% SUVmax threshold, pts obtaining CR after salvage chemotherapy exhibited lower baseline values vs. those obtaining PR as evidenced by MTV (median 20 vs. 88 ml, P<0.001), TLGmax (88.5 vs. 411.2, P<0.001) and TLG global (142.1 vs. 828.2, P<0.001). TLG performed better in identifying pts obtaining CR vs. those presenting with progression of disease after salvage chemotherapy (TLGmax: 88.4 vs. 491.6, P=0.043; TLG global: 142.1 vs. 946. 7; P=0.034). Baseline SUVmax was similar in CR and PR (median 13.6 vs. 15.5, P=0.467) pts.
Implementing logistic regression models, MTV ≥ 54.2ml pre-salvage chemotherapy was a significant predictor of failure to achieve CR (Odds ratio [OR]= 7.89 (95%CI: 2.58-24.16); P<0.001; AUC_ROC=0.723). MTV retained its prognostic value even after adjustment with sAAIPI (OR= 7.11 (95%CI: 2.29-22.09); P<0.001; AUC_ROC: 0.748).
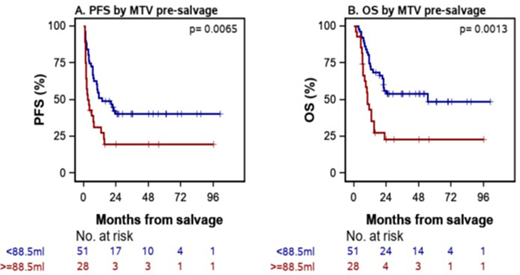
As analyzed by intent to treat, with a median follow up of 18.8 months, the Kaplan-Meier estimates of the proportion of pts alive and progression-free (PFS) and of the overall survival (OS) at 2 years were 32.8% (95%CI: 22.6% to 43.5%) and 43.2% (95%CI: 31.8% to 54.1%), respectively. In models including one PET/CT metric variable and adjustment for sAAIPI, pre-salvage therapy MTV ≥ 88.5ml and TLGmax ≥ 43.2 were significant predictors of worse PFS (HR=1.91, P=0.026 and HR=2.20, P=0.033) and OS (HR=2.26, P=0.012 and HR =2.88, P=0.017), respectively. Figures A and B show Kaplan-Meier plots for PFS and OS by low-high MTV groups.
Conclusions: Pre-salvage MTV appropriately identified pts with r/r DLBCL treated with platinum-based chemotherapy at risk for treatment failure. Furthermore, MTV and TLGmax identified a high-risk group of pts associated with shorter survival. This data can be of used to determine which pts achieve maximal benefit with a standard program of 2-3 cycles of salvage chemotherapy followed by ASCT. Updated results on salvage therapy response and survival analysis will be presented at the meeting. Prognostic value of PET/CT metrics in r/r DLBCL should be confirmed in prospective clinical studies with the ultimate goal of developing strategies for an individualized approach.
Alderuccio:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Other: Family member; Oncinfo: Honoraria; Inovio Pharmaceuticals: Other: Family member; Foundation Medicine: Other: Family member; OncLive: Honoraria; Forma Therapeutics: Other: Family member; Puma Biotechnology: Other: Family member. Beitinjaneh:Kite, a Gilead Company: Honoraria, Other: consulting or advisory role, speaker's bureau, ; Jazz: Other: speaker's bureau; Gilead: Research Funding; ATARA: Research Funding. Lossos:Seattle Genetics: Consultancy, Other; Janssen Scientific: Consultancy, Other; Verastem: Consultancy, Honoraria; Janssen Biotech: Honoraria; NCI: Research Funding; Stanford University: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.