Abstract
Anemia is a very common comorbidity in patients with heart failure (HF), affecting ∼30% of stable ambulatory patients and 50% patients with acute decompensated HF. Absolute or functional iron deficiency (ID) is seen in ∼50% patients with HF. Both of these comorbidities often coexist and are independently associated with increased mortality and hospitalizations. These findings led several investigators to test the hypotheses that treatment of anemia and ID in HF would improve symptoms and long-term outcomes. Small studies showed that erythropoiesis-stimulating agents (ESAs) improve subjective measures of HF. However, a large pivotal outcome trial found that the ESA darbepoetin alfa did not improve long-term outcomes in patients with HF with reduced ejection fraction and instead was associated with adverse effects. Studies using IV iron have had somewhat greater success, showing improvements in subjective and some objective measures of HF. However, more research is needed to establish the best treatment options for these high-risk patients. We present 5 common scenarios of patients with HF and anemia and describe our personal approach on how we might treat them based on objective evidence where available. An algorithm that offers guidance in regard to personalized therapy for such patients is also presented.
Introduction
Anemia is common in patients with heart failure (HF) and reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF) and is associated with worse long-term outcomes.1-6 The pathogenesis of anemia in HF is multifactorial (reviewed in detail in Anand and Gupta1 ). Vitamin B12 or folate deficiencies are relatively infrequent (∼4% to 5%), but iron deficiency (ID) is extremely common.7-10 Other important contributors include neurohormonal and proinflammatory cytokine activation and renal dysfunction.1 Patients with severe anemia often have features of worse HF with more extensive left ventricular (LV) remodeling and higher levels of biomarkers of advanced HF, higher inflammatory and collagen markers, and worse renal function. These factors lead to the development of anemia of chronic disease with defective iron utilization, inappropriate erythropoietin responsiveness, and depressed bone marrow function. However, mechanisms by which anemia worsens HF are not entirely clear. In patients with normal LV function, very severe anemia (hemoglobin [Hb] ∼4 to 6 g/dL) can cause the syndrome of high-output HF that resolves completely with an increase in Hb.3,11,12 In patients with HFrEF, anemia may increase myocardial workload to compensate for reduced tissue oxygen delivery and worsen unfavorable cardiac remodeling. Other comorbidities seen in patients with HF, including diabetes mellitus, chronic kidney disease (CKD), and hypertension, also worsen HF outcomes. In patients with CKD, anemia is partly related to inappropriate erythropoietin production and response. In patients with CKD and moderate anemia (Hb ∼8.5 g/dL), increasing the Hb level (up to 14 g/dL) with erythropoiesis-stimulating agents (ESAs) progressively decreased left ventricular ejection fraction (LVEF) and cardiac output,13 probably because a higher hematocrit value leads to increased blood viscosity and decreased nitric oxide availability, thereby raising systemic vascular resistance and afterload.12,14 Despite the comorbidities, anemia remains an independent predictor of mortality and morbidity in patients with chronic HFrEF or HFpEF or acute decompensated HF.1,2
ID in HF can be absolute, when total body iron is decreased (“low iron storage”), or functional, when total body iron is normal or increased but inadequate to meet the needs of target tissues. This results from sequestration of iron in the storage pool (defective iron utilization or “reticuloendothelial block”), a characteristic of anemia of chronic disease/inflammation (Figure 1).1,15,16 Recent trials of IV iron supplementation in HF defined ID as a serum ferritin level <100 μg/L, or 100 to 300 μg/L with transferrin saturation (TSAT) <20%.17-20 This definition was subsequently shown to correlate with bone marrow ID21 and is now endorsed by most HF guidelines.22,23 However, a definition solely based on either TSAT ≤ 19.8% or serum iron ≤13 μmol/L had an even higher sensitivity and specificity for ID compared with the aforementioned definition, based on bone marrow iron status, considered the gold standard for ID.21 This definition also identified HF patients at the highest risk of death, whereas ferritin was not independently associated with mortality. Thus, although this study validates the currently used TSAT cutoff of <20% in HF patients, it questions the value of ferritin for initial assessment of ID in this setting.
Absolute and functional ID. ID can be absolute, when total body iron is decreased, or functional, when total body iron is normal or increased but sufficient iron is not available to target tissues because of iron sequestration in the storage pool (iron maldistribution). Both storage and functional pools are smaller in absolute ID, whereas only the functional pool is reduced in functional ID. Either condition can occur independently or coexist in an individual patient. Absolute ID in HF can occur due to reduced intake because of anorexia, cardiac cachexia, impaired iron absorption because of intestinal edema, and hepcidin-induced downregulation of iron transporters such as ferroportin. Other causes include gastrointestinal blood losses related to use of aspirin, antiplatelet agents, or anticoagulants, or important coexisting conditions, such as malignancies of the gastrointestinal or genitourinary tract.24,42,63-65 Functional ID in HF results from mechanisms similar to those responsible for the anemia of chronic disease or inflammation.16,66 HF is associated with increased levels of inflammatory cytokines, including interleukin (IL)-1, IL-6, IL-18, and tumor necrosis factor-α (TNF-α). These cytokines, particularly IL-6, upregulate hepatic hepcidin production via JAK/Stat3, which binds, internalizes, and degrades ferroportin. This results in impairment of iron absorption into the blood from enterocytes and entrapment of iron in the storage pool (liver and reticuloendothelial cells [RES]). Together, these effects result in relative iron depletion in erythroid cells as well as nonerythroid tissues (functional pool). Inflammatory cytokines also blunt renal erythropoietin production and erythroblast responsiveness to erythropoietin. Erythroblast proliferation is also directly inhibited by elevated levels of hepcidin, further impairing Hb synthesis. Tf, transferrin. Reprinted from Anand and Gupta1 with permission.
Absolute and functional ID. ID can be absolute, when total body iron is decreased, or functional, when total body iron is normal or increased but sufficient iron is not available to target tissues because of iron sequestration in the storage pool (iron maldistribution). Both storage and functional pools are smaller in absolute ID, whereas only the functional pool is reduced in functional ID. Either condition can occur independently or coexist in an individual patient. Absolute ID in HF can occur due to reduced intake because of anorexia, cardiac cachexia, impaired iron absorption because of intestinal edema, and hepcidin-induced downregulation of iron transporters such as ferroportin. Other causes include gastrointestinal blood losses related to use of aspirin, antiplatelet agents, or anticoagulants, or important coexisting conditions, such as malignancies of the gastrointestinal or genitourinary tract.24,42,63-65 Functional ID in HF results from mechanisms similar to those responsible for the anemia of chronic disease or inflammation.16,66 HF is associated with increased levels of inflammatory cytokines, including interleukin (IL)-1, IL-6, IL-18, and tumor necrosis factor-α (TNF-α). These cytokines, particularly IL-6, upregulate hepatic hepcidin production via JAK/Stat3, which binds, internalizes, and degrades ferroportin. This results in impairment of iron absorption into the blood from enterocytes and entrapment of iron in the storage pool (liver and reticuloendothelial cells [RES]). Together, these effects result in relative iron depletion in erythroid cells as well as nonerythroid tissues (functional pool). Inflammatory cytokines also blunt renal erythropoietin production and erythroblast responsiveness to erythropoietin. Erythroblast proliferation is also directly inhibited by elevated levels of hepcidin, further impairing Hb synthesis. Tf, transferrin. Reprinted from Anand and Gupta1 with permission.
Both absolute and functional ID (with or without anemia) are extremely common, occurring in up to 50% (or even more in some studies) of patients with HF.7-9,24 ID is also independently related to worse outcomes, but the underlying mechanisms are not entirely clear.25 Besides the need for iron in Hb, iron is an essential component of mitochondrial enzymes. Systemic ID is associated with reduced myocardial iron content and causes mitochondrial and LV dysfunction via several mechanisms.26-29 ID reduces exercise capacity and quality of life (QoL) in both HFrEF and HFpEF and increases mortality, independent of anemia.7,9,30-34 IV iron administration in selected patients with HFrEF (LVEF <45%, New York Heart Association [NYHA] class II to III, with or without anemia [Hb <15 g/dL] with absolute/functional ID [ferritin level <100 μg/L or ferritin level 100 to 300 μg/L with TSAT <20%]) and ID improves clinical outcomes in both anemic and nonanemic patients.1 Moreover, IV iron improved skeletal muscle mitochondrial function in HFrEF patients with ID, independent of anemia.35 The adverse clinical consequences of ID in patients with HFrEF could therefore be related to direct effects on myocardial and skeletal muscle function and could improve with IV iron administration.
Correcting anemia and ID in patients with HF would, therefore, appear to be important therapeutic targets to improve long-term outcomes. Several small clinical trials and meta-analyses studied the effects of increasing Hb with different ESAs. One meta-analysis examined the effects of ESAs in 11 small studies on 794 patients with HFrEF and anemia.36 ESAs increased Hb by ∼2 g/dL and was associated with significant improvements in NYHA class, exercise duration, peak oxygen consumption, 6-minute walk distance (6MWD), and QoL, compared with controls. HF-related hospitalizations were reduced by 44% (P = .005), but reduction in all-cause mortality was of borderline significance. However, a large, more definitive and pivotal Reduction of Events by Darbepoetin Alfa in Heart Failure trial found that treating anemic patients with an ESA did not improve clinical outcomes in patients with HFrEF and mild to moderate anemia (Hb, 9.0 to 12.0 g/dL), but was associated with a significantly higher incidence of thromboembolic events and a nonsignificant increase in strokes.37 These data therefore do not support the use of ESAs in HF.
Oral iron supplementation is the standard therapy for patients with ID because it is convenient, readily available, and inexpensive. However, in patients with HF, apart from gastrointestinal intolerance, oral iron is poorly absorbed because of elevated hepcidin, which inhibits iron absorption by reducing transmembrane ferroportin on enterocytes, thereby preventing iron transfer from enterocytes to blood.1,16 Few studies have examined the effects of oral iron in patients with ID and HF.38 Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT HF) was the largest randomized study of high-dose oral iron in patients with HF.39 Patients (n = 225) with NYHA class II to IV HF (median LVEF, 25%), Hb 9 to 15 g/dL (men) or 9 to 13.5 g/dL (women), and ID (ferritin level 15 to 100 μg/L or 100 to 299 μg/L with TSAT < 20%) received either oral iron polysaccharide or placebo. At 16 weeks, there was no significant difference between the groups in the primary end point of change in peak oxygen consumption from baseline or in any secondary end point (6MWD, N-terminal pro–B-type natriuretic peptide levels, or QoL), although oral iron increased TSAT, ferritin, and hepcidin and reduced soluble transferrin receptor-1 levels. Based on these results, oral iron replacement is not indicated in this setting.
The role of IV iron in HF has been studied for over 10 years. A number of small and medium-sized uncontrolled studies and randomized control trials (RCTs) reported the effects of IV iron in patients with HFrEF.1 The primary objective of these studies was to investigate the safety and efficacy of IV iron on exercise capacity, NYHA class, and QoL. Clinical events were recorded as safety secondary outcomes. Of the randomized trials published to date, 3 trials (n = 98 patients) used IV iron sucrose and 3 trials (n = 935 patients) used ferric carboxymaltose (FCM).17-20,38,40 Collectively, these studies showed that IV iron has beneficial effects on most subjective outcomes, including NYHA class, 6MWD, patient global assessment, QoL, and fatigue score (supplemental Table 1, available on the Blood Web site). However, the hard endpoint of all-cause mortality was not affected, and there was borderline improvement in hospitalizations for HF.
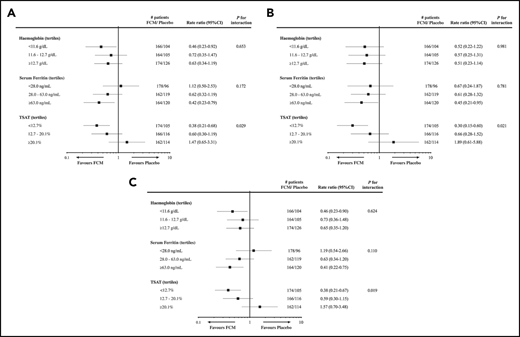
A recent meta-analysis reported the effects of IV FCM on mortality and hospitalizations using individual patient data from 4 RCTs, comparing FCM with placebo in 839 patients with HFrEF and ID.41 This analysis showed that FCM treatment is associated with lower rates of recurrent cardiovascular hospitalizations and cardiovascular mortality (rate ratio, 0.59; 95% confidence interval [CI], 0.40 to 0.88; P = .009), recurrent HF hospitalizations and cardiovascular mortality (rate ratio, 0.53; 95% CI, 0.33 to 0.86; P = .011), and recurrent cardiovascular hospitalizations and all-cause mortality (rate ratio, 0.60; 95% CI, 0.41 to 0.88; P = .009). IV iron was well tolerated. However, a prespecified subgroup analysis found a significant interaction between TSAT tertiles and treatment effect on all 3-composite outcomes, with the greatest benefit seen in the lowest TSAT tertile (<12.7%) with no benefit in subgroup with TSAT of 12.7% to 20.1%, and a trend to adverse effects of IV iron in the highest TSAT tertile (≥20.1%) (Figure 2). Further in-depth analysis of these data found that FCM reduced cardiovascular hospitalizations and cardiovascular mortality only in patients with TSAT ≤19.8%.21 Therefore, although these findings support the short-term use of IV iron in patients with absolute or functional ID, they also raise concerns about the deleterious effects of overcorrecting ID in HF, particularly over prolonged periods. Further studies are required to address these concerns.
Subgroup analyses for outcomes after administration of IV iron, by baseline tertiles of Hb, serum ferritin, and TSAT. Subgroup analyses for recurrent cardiovascular hospitalizations and cardiovascular mortality (A), recurrent HF hospitalizations and cardiovascular mortality (B), and recurrent cardiovascular hospitalizations and all-cause mortality (C), from the individual patient data meta-analysis of 4 studies examining the effects of FCM in iron-deficient HF patients. Reprinted from Anker et al41 with permission.
Subgroup analyses for outcomes after administration of IV iron, by baseline tertiles of Hb, serum ferritin, and TSAT. Subgroup analyses for recurrent cardiovascular hospitalizations and cardiovascular mortality (A), recurrent HF hospitalizations and cardiovascular mortality (B), and recurrent cardiovascular hospitalizations and all-cause mortality (C), from the individual patient data meta-analysis of 4 studies examining the effects of FCM in iron-deficient HF patients. Reprinted from Anker et al41 with permission.
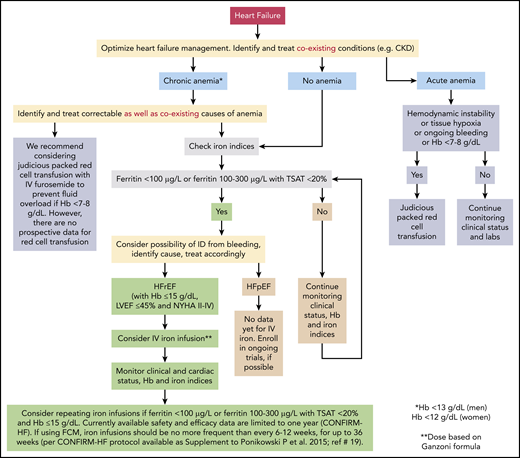
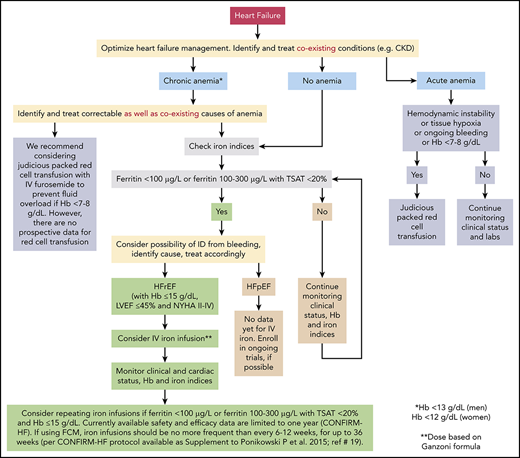
We present 5 representative cases to discuss our approach to management of anemia in different clinical scenarios. We also propose an algorithm for managing anemia and ID in patients with HF, based on currently available evidence (Figure 3). Furthermore, we emphasize that any investigation of anemia in patients with HF should begin with identification and treatment of correctable causes of anemia (including blood loss in those with ID).10,24,42
Evidence-based approach to treating anemia and ID in patients with HF. The figure represents our recommended approach to evaluation and treatment of anemia and/or ID in patients with HF. Supporting evidence, where available, is discussed in detail in the text. Since trials of IV iron did not include patients with Hb >15 g/dL, LVEF >45%, or NHYA class I, we do not recommend administering IV iron to such patients. Data for IV iron in patients with NYHA class IV are limited. There are no currently available prospective data on the safety and efficacy of IV iron administration beyond the last time point in the CONFIRM-HF trial (36 weeks); therefore, we do not recommend routinely administering IV iron beyond 36 weeks. See Table 1 for information and discussion about various IV iron preparations. In 1 study, the prevalence of intestinal malignancies was similar in iron-deficient HF patients with or without anemia (9.3% vs 10.5%, respectively; P = .55). Prevalence was lower in HF patients without ID, with or without anemia (6% and 1.5%, respectively).42 In a different population, gastrointestinal malignancies were found in 2.6% and 1.5% patients with and without anemia, respectively.24 In our opinion, HF patients with ID (with or without anemia) should be evaluated for possible sources of bleeding; those who are anemic but not iron deficient should be evaluated on a case-by-case basis. Routine age-, sex-, and risk-based cancer screening remains appropriate for all patients.
Evidence-based approach to treating anemia and ID in patients with HF. The figure represents our recommended approach to evaluation and treatment of anemia and/or ID in patients with HF. Supporting evidence, where available, is discussed in detail in the text. Since trials of IV iron did not include patients with Hb >15 g/dL, LVEF >45%, or NHYA class I, we do not recommend administering IV iron to such patients. Data for IV iron in patients with NYHA class IV are limited. There are no currently available prospective data on the safety and efficacy of IV iron administration beyond the last time point in the CONFIRM-HF trial (36 weeks); therefore, we do not recommend routinely administering IV iron beyond 36 weeks. See Table 1 for information and discussion about various IV iron preparations. In 1 study, the prevalence of intestinal malignancies was similar in iron-deficient HF patients with or without anemia (9.3% vs 10.5%, respectively; P = .55). Prevalence was lower in HF patients without ID, with or without anemia (6% and 1.5%, respectively).42 In a different population, gastrointestinal malignancies were found in 2.6% and 1.5% patients with and without anemia, respectively.24 In our opinion, HF patients with ID (with or without anemia) should be evaluated for possible sources of bleeding; those who are anemic but not iron deficient should be evaluated on a case-by-case basis. Routine age-, sex-, and risk-based cancer screening remains appropriate for all patients.
Case 1: anemia without absolute or functional ID in a patient with HF
A 71-year-old man was referred for evaluation and management of chronic, stable anemia. He had well-compensated NYHA class II HFrEF with mild exertional dyspnea, no chest pain or palpitations. Recent LVEF was 30%. There was no history of bleeding. Recent screening colonoscopy was normal. His blood pressure was 122/78 mm Hg and his pulse was 77 bpm with no signs of fluid retention.
Laboratory investigations confirmed anemia (Hb 11.2 g/dL). The mean corpuscular volume (MCV), white blood cell count (WBC), differential and platelet counts, and serum creatinine, B12, and folate levels were normal. TSAT was 25% (normal range, ∼15% to 50%), and ferritin level was 315 μg/L (normal, 24 to 336 μg/L in men and 11 to 307 μg/L in women). Peripheral blood smear was unremarkable. There was no morphological or biochemical evidence of hemolysis. An electrocardiogram was normal apart from nonspecific ST- and T-wave changes in the lateral chest leads. The chest X-ray (CXR) was normal. HF medications included lisinopril 15 mg/d, carvedilol 12.5 mg twice daily, spironolactone 12.5 mg/d, and furosemide 40 mg/d. Cardiology consultation had recommended optimizing HF management, increasing carvedilol to 25 mg twice daily, and switching lisinopril to sacubitril-valsartan and up-titrating as tolerated. His primary physician requested a hematology consultation to evaluate whether he might be a good candidate to receive an ESA.
Case 1 discussion
Our patient, with no identifiable cause of anemia, has anemia of chronic disease, which is commonly seen in HF.37 In patients with both HFrEF and HFpEF, anemia is independently associated with worse cardiovascular outcomes, including hospitalizations and mortality.1-6 Therefore, for years, it was considered that correction of anemia to near-normal levels in patients with HF would improve symptoms, QoL, and clinical outcomes. However, publication of the Reduction of Events by Darbepoetin Alfa in Heart Failure trial in 2013 made it clear that treatment of anemia with an ESA did not improve any clinical outcome but was associated with a higher incidence of thromboembolic events.37 Similar increases in adverse events, including death, occur when the Hb is raised to near-normal levels with ESAs in patients with CKD, with or without HF.43-47 ESAs should therefore not be used to increase Hb to normal or near-normal levels in HF patients with mild to moderate anemia. In patients with CKD, the Food and Drug Administration and Kidney Disease: Improving Global Outcomes guidelines recommend initiating ESAs when Hb <10 g/dL, and withholding ESAs when Hb ≥11 g/dL.48,49 In keeping with HF guidelines, this patient should undergo a diagnostic workup to identify and treat correctable causes of anemia, but should not be treated with ESAs to improve outcomes.22 Complete blood count and iron parameters should be monitored regularly.
No prospective RCTs have examined whether red cell transfusion (transfusion) is of benefit in HF patients with chronic anemia in the outpatient setting. Our patient’s Hb is considerably above the transfusion threshold recommended by the American Association of Blood Banks (7 to 8 g/dL),50 and transfusion carries well-known risks. Our patient also does not have absolute/functional ID. In patients with TSAT >20%, IV iron was associated with possible harm.21,41 This patient should therefore not receive transfusion or IV iron.
Case 2: anemia with functional ID in a patient with HF
The patient described in case 1 returned 2 years later. He had been getting more dyspneic and easily fatigued (NYHA class III) and required an extra dose of furosemide 40 mg every few days. LVEF had worsened to 20%. There was no history of bleeding; stool sample was negative for occult blood. There was no history of acute or chronic infections, autoimmune diseases, malignancies, or recent trauma. His blood pressure was 108/70 mm Hg; pulse was 70 bpm, and there were no signs of fluid retention.
Laboratory investigations confirmed anemia (Hb 9.0 g/dL). The MCV, WBC, differential and platelet counts, serum creatinine, B12, and folate levels remained normal. Iron parameters now showed TSAT at 14% and ferritin level at 200 μg/L. Peripheral blood smear examination showed normocytic anemia, but no other morphological abnormalities. The electrocardiogram and CXR remained unchanged. HF medications included carvedilol 25 mg twice daily, spironolactone 25 mg/d, sacubitril-valsartan 97/103 mg twice daily, and furosemide 40 mg twice daily, and an extra dose whenever required.
Case 2 discussion
The patient now meets therapeutically relevant criteria for anemia with absolute or functional ID (ferritin level <100 μg/L, or TSAT <20% with ferritin level 100 to 300 μg/L).17-20,41 In patients undergoing coronary artery bypass grafting, when sternal bone marrow could be easily sampled, these parameters correlated well with bone marrow ID.21 In this study, TSAT ≤ 19.8% or serum iron ≤13 μmol/L (≤72.6 μg/dL) as single parameters also correlated well with absolute or functional bone marrow ID. Notably, patients with ferritin level <100 μg/L and TSAT > 20% did not have bone marrow ID, suggesting that a low TSAT may be a more reliable indicator of ID in HF.
Our patient now has a clear indication for starting iron replacement. The beneficial effects of IV iron administration in patients with HFrEF and ID are summarized above and in supplemental Table 1. In the largest randomized study (FAIR-HF), patients with HFrEF and ID (defined as ferritin level <100 μg/L, or TSAT < 20% with ferritin level 100 to 300 μg/L) with or without anemia received FCM or saline.17 At 6 months, although ferritin increased in all patients, only anemic patients experienced a small increase in Hb (0.9 g/dL). However, FCM significantly improved patients’ global assessment, NYHA class, QoL, and 6MWD in anemic as well as nonanemic patients. FCM did not improve first hospitalizations or mortality. However, the meta-analysis of individual patient data from 4 RCTs of FCM vs placebo in patients with ID and HFrEF, mentioned above, found that FCM administration was associated with reduction in the relevant hard outcomes of mortality and HF hospitalization.41 Therefore, it is now advisable to offer IV iron (Table 1) to this symptomatic patient, rather than only continuing to monitor symptoms, blood counts, and iron parameters.
It remains to be determined if the clinical benefits of IV iron administration are sustained for longer periods (>1 year),19 and what the potential long-term risks of repeated administration of IV iron (eg, iron overload) may be.1 Until such information is available, we believe that this treatment can be offered to selected patients while continuing to monitor cardiac status, blood counts, and iron parameters closely, exercising particular caution in administering repeated large doses of IV iron.
Oral iron supplementation is unlikely to be of clinical benefit even if iron parameters improve, as shown in a post hoc analysis of the IRONOUT-HF study.39,51 Although the patient is anemic, ESAs are unlikely to improve clinical outcomes and may cause adverse outcomes, particularly if a higher Hb level is targeted.37
Case 3: anemia with absolute ID in a patient with HF
A 38-year-old woman was referred for management of ID anemia that had not improved despite treatment with oral iron supplementation for 3 months. She had been compliant with and tolerated immediate release ferrous sulfate thrice daily. Menstrual history was normal. She had 2 children, 14 and 11 years old. Recent gastrointestinal evaluation did not identify any source of bleeding; evaluation for celiac disease was negative. Past history was notable only for well-compensated HFrEF, NYHA class II, LVEF 35%, which developed 5 years ago after a febrile “viral” illness. She reported dyspnea walking 1 block.
Laboratory evaluation confirmed microcytic anemia (Hb 8.7 g/dL, MCV 69 fL, and red blood cell count 3.0 × 106/μL). WBC, differential and platelet counts, and serum creatinine, B12 and folate levels were normal. TSAT was 9%, and ferritin level was 21 μg/L and had not improved with oral ferrous sulfate. Peripheral blood smear examination confirmed microcytic, hypochromic anemia. The LVEF remained unchanged after oral iron supplementation.
Case 3 discussion
This patient clearly has absolute ID,21 a condition that usually responds to oral iron supplementation. However, for reasons that are not entirely clear, patients with HFrEF do not respond well to oral iron, as shown in the IRONOUT-HF trial.39,51 Elevated hepcidin levels in inflammatory conditions, including HF, reduce enterocyte membrane ferroportin and consequently inhibit iron absorption.16,39 Notably, although oral iron modestly increases TSAT and ferritin in patients with low hepcidin levels, there is no association between response to oral iron supplementation and clinical benefit.39,51
Repletion of iron stores and improvement in hematological and clinical parameters are likely to be achieved in this patient by administration of adequate doses of IV iron (Table 1).19,52 Complete blood count and iron parameters will need to be monitored serially to confirm response and determine the need for repeated administration of IV iron. Oral iron supplementation should be discontinued because it was ineffective and may cause gastrointestinal and other adverse effects. Although changing to a different oral iron formulation may improve tolerance and compliance in patients experiencing adverse effects from the initial formulation, it is unlikely to be of benefit in the setting of HF. Because the impairment of iron absorption in HF is a consequence of the effects of hepcidin, it is also unlikely that coadministration of vitamin C will adequately increase gastrointestinal iron absorption.
In patients with HF and absolute ID, the prevalence of underlying gastrointestinal malignancies is substantial and is reported to be similar in those with or without anemia (9.3% vs 10.5%, respectively).42 We therefore recommend evaluating patients with HF and absolute ID for a source of bleeding, irrespective of anemia.
Case 4: anemia due to an unrelated comorbidity in a patient with HF
A 67-year-old postmenopausal woman was referred for evaluation and management of anemia. She had well-compensated NYHA class II HFrEF but had started feeling increasingly dyspneic on exertion over the past 6 months. She did not smoke or drink alcohol. There was no history of bleeding. LVEF was 40%, unchanged from 1 year ago, and CXR was normal.
Laboratory investigations demonstrated anemia (Hb 9.7 g/dL with MCV 112 fL; values 1 year earlier were Hb 12.3 g/dL and MCV 98 fL). WBC, differential and platelet counts, reticulocyte count, thyrotropin, liver function tests, and serum creatinine, B12, and folate levels were normal. TSAT was 16%, and ferritin level was 225 μg/L. Peripheral blood smear examination showed macrocytic erythrocytes and a population of bilobed neutrophils lacking normal cytoplasmic granulation.
Bone marrow biopsy revealed multilineage dysplasia, 7% blasts, and normal cytogenetics, consistent with myelodysplastic syndrome (MDS with excess blasts; Revised International Prognostic Scoring System [IPSS-R] risk category, intermediate).53,54 Some stainable iron was present in the bone marrow. She received treatment with subcutaneous azacitidine, which resulted in improvement in symptoms and Hb.55,56
Case 4 discussion
IV iron could otherwise be considered in the setting of HFrEF and iron parameters consistent with functional ID,15,19,21 but in this patient, it is critical to diagnose and treat the MDS that is the cause of recent progressive anemia and carries significant risk of transformation to acute myeloid leukemia.53 It is important for physicians to remain vigilant for the presence of other diseases or coexisting conditions that can cause or contribute to anemia in patients with HF. In this patient, “red flags” included the recent change in symptoms despite stable cardiopulmonary status, relatively rapid decline in Hb, and the onset of macrocytosis. Examination of peripheral blood morphology should always be undertaken when evaluating anemia, because it may identify abnormalities suggesting the presence of several other causes of anemia.
Particular caution will need to be exercised when deciding to administer IV iron in this patient, because hematological conditions such as MDS can cause deleterious iron overload in the long term.57 Although administration of an ESA can increase Hb levels in low-risk MDS,58 it is not an appropriate treatment of MDS with multilineage dysplasia and increased blast percentage. In a patient such as this, the median time to transformation to acute myeloid leukemia is short,53 which can be delayed by treatment with hypomethylating agents such as azacitidine or decitabine but not by ESAs.56
Case 5: acute postoperative anemia in a patient with HFrEF
An inpatient hematology consultation was requested for management of anemia in an 80-year-old woman who underwent coronary artery bypass grafting and bioprosthetic aortic valve replacement 7 days previously. Cardiac history was notable for HFrEF (NYHA III, LVEF 35%), dyspnea, and angina with minimal exertion, with 3-vessel coronary artery disease and severe aortic stenosis. Prior to surgery, Hb was 12.5 g/dL. WBC, differential, platelet counts, and creatinine level were normal. Other than development of postoperative anemia (Hb 8.3 g/dL, which had stabilized over the previous 3 days), she was asymptomatic, recovering uneventfully on a surgical ward. Pulse was 85 bpm; blood pressure was 115/70 mm Hg, and respiratory rate was 14/min. The patient was concerned that she was not receiving transfusions to restore the considerable drop in Hb.
Case 5 discussion
An ∼4 g/dL drop in Hb after cardiac surgery can be cause for concern. Hemodynamic stability, adequacy of peripheral perfusion, and tissue oxygenation need to be assured, and ongoing bleeding or hemolysis excluded. Thereafter, routine transfusions may expose patients to the diverse risks of blood product transfusions without necessarily improving symptoms or outcomes. The Transfusion Requirements in Cardiac Surgery III noninferiority trial randomized adults with a moderate to high risk of death following cardiac surgery to a restrictive or liberal transfusion strategy during and after surgery.59 Patients in the restrictive strategy group received transfusion if Hb <7.5 g/dL at any time, whereas those in the liberal strategy group were transfused if Hb <9.5 g/dL intraoperatively or in the intensive care unit or <8.5 g/dL after being transferred out of the intensive care unit. The mean age was 72 ± 10 years, and baseline mean Hb was 13.1 g/dL in both groups. About 38% patients had reduced LVEF (≤50%), and 54% patients had impaired renal function. Among patients in the restrictive strategy group, 47.7% did not require any transfusion, compared with 27.4% in the liberal strategy group. Median hospital stay was 8 days in both groups. The primary composite outcome (death, myocardial infarction, stroke, or new onset kidney failure requiring dialysis) occurred in 11.4% and 12.5% patients in the restrictive and liberal strategy groups, respectively (P < .001 for noninferiority). Among patients ≥75 years, the risk of the primary composite outcome was lower (rather than higher) in the restrictive transfusion group, providing reassurance that this strategy is not detrimental for older patients. A previous trial (Transfusion Indication Threshold Reduction) randomized patients undergoing cardiac surgery postoperatively to restrictive vs liberal transfusion strategy had reported that the secondary outcome of mortality was higher in the restrictive strategy group.60 The 2016 American Association of Blood Banks clinical practice guidelines (published before Transfusion Requirements in Cardiac Surgery III) recommend a similar threshold of withholding transfusions until Hb 7 g/dL in hemodynamically stable hospitalized adults and 8 g/dL for adults undergoing orthopedic or cardiac surgery or those with preexisting cardiovascular disease.50 A more recent meta-analysis of 13 RCTs in patients undergoing cardiac surgery reported that restrictive transfusion was noninferior to liberal transfusion for risk of myocardial infarction, stroke, renal failure, infection, or death within 30 days of surgery.61
Therefore, in this asymptomatic patient with a stable Hb, it is appropriate to provide reassurance and to continue to monitor symptoms, hemodynamic stability, and Hb level, and only administer transfusion if she develops symptoms related to the anemia, becomes hemodynamically unstable with tissue hypoxia, or if the Hb declines to <7.5 g/dL. There is no indication for administration of IV iron or ESAs.
Conclusions
Anemia and ID (absolute or functional) are very common comorbidities in patients with HF and are associated with poor clinical status and worse outcomes. Although the causes of anemia in HF are not entirely clear, neurohormonal and proinflammatory cytokine activation and renal dysfunction favor development of anemia of chronic disease/inflammation. ESAs do not improve outcomes, may be associated with thromboembolic complications, and are not recommended. In patients with HFrEF and absolute or functional ID with or without anemia, IV but not oral iron therapy may improve symptoms and outcomes. Ongoing larger, adequately powered trials with cardiovascular mortality and morbidity end points will determine the long-term efficacy and safety of IV iron in patients with HF. Concerns that overcorrection of ID could be deleterious need to be carefully addressed. IV iron should not be administered during active infections.
Although patients with HFpEF have similar prevalence of anemia and ID that is similarly associated with poor clinical status and worse outcomes, benefits of these therapies have not been tested in these patients. Fortunately, numerous long-term ongoing studies are likely to provide valuable information to address these concerns and to help guide clinical decision making. Several IV iron preparations are available for clinical use (Table 1). We hope that novel pathophysiologically based approaches being developed for treatment of anemia of inflammation, including those targeting the hepcidin pathway or hypoxia-inducible factor prolyl hydroxylases, will expand and improve therapeutic options.16,62
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Thomas D. Coates (Children’s Hospital Los Angeles, University of Southern California, Los Angeles, CA) and Gordon D. McLaren (VA Long Beach Healthcare System, Long Beach, CA and University of California Irvine, Irvine, CA) for their insightful review of the manuscript and helpful suggestions.
Authorship
Contribution: I.A. and P.G. conceived and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Inder Anand, University of Minnesota Medical School, 5448 Caminito Bayo, La Jolla, CA 92037; e-mail: anand001@umn.edu.

![Absolute and functional ID. ID can be absolute, when total body iron is decreased, or functional, when total body iron is normal or increased but sufficient iron is not available to target tissues because of iron sequestration in the storage pool (iron maldistribution). Both storage and functional pools are smaller in absolute ID, whereas only the functional pool is reduced in functional ID. Either condition can occur independently or coexist in an individual patient. Absolute ID in HF can occur due to reduced intake because of anorexia, cardiac cachexia, impaired iron absorption because of intestinal edema, and hepcidin-induced downregulation of iron transporters such as ferroportin. Other causes include gastrointestinal blood losses related to use of aspirin, antiplatelet agents, or anticoagulants, or important coexisting conditions, such as malignancies of the gastrointestinal or genitourinary tract.24,42,63-65 Functional ID in HF results from mechanisms similar to those responsible for the anemia of chronic disease or inflammation.16,66 HF is associated with increased levels of inflammatory cytokines, including interleukin (IL)-1, IL-6, IL-18, and tumor necrosis factor-α (TNF-α). These cytokines, particularly IL-6, upregulate hepatic hepcidin production via JAK/Stat3, which binds, internalizes, and degrades ferroportin. This results in impairment of iron absorption into the blood from enterocytes and entrapment of iron in the storage pool (liver and reticuloendothelial cells [RES]). Together, these effects result in relative iron depletion in erythroid cells as well as nonerythroid tissues (functional pool). Inflammatory cytokines also blunt renal erythropoietin production and erythroblast responsiveness to erythropoietin. Erythroblast proliferation is also directly inhibited by elevated levels of hepcidin, further impairing Hb synthesis. Tf, transferrin. Reprinted from Anand and Gupta1 with permission.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/7/10.1182_blood.2019004004/3/m_bloodbld2019004004cf1.png?Expires=1765306118&Signature=uwuzDM5X4f2mFJEthCp~lQa0AWBaYc0U5n9ujYTQmXmpzQ~ECkfh-xbg~NCFsbZpZRNDLdq7Fw1SO5iZK1tP~QUtbuyf~ZeYUU5PEs5W-tlvi5WoAF0Mm7d2jIUsS9SY3EjgUEfYEgk8EmLUzQCTiQWHKju~KKsSkzHnhUvxrJoh92toV3EqYci1TbLU1PYt~yLw0DMTBMoChCB9Kwwa8cV47-YtQB72zZB~nannjx58RJmEITYZuON5lvPCWUmpJu4yyvu4GOFsv4zAfsZKS-ufRegBF~sjkjb6FvzEMvPFyOF1kojMnN39rnPO8ebMRMfZCQxEHZgp-ZkxqNDyig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)