Signaling through the CD27/CD70 pathway is critical for Epstein-Barr virus (EBV) immune surveillance, and, in this issue of Blood, Ghosh et al report on symptoms and treatment of an international cohort of patients with defects in CD27 or its ligand, CD70.1
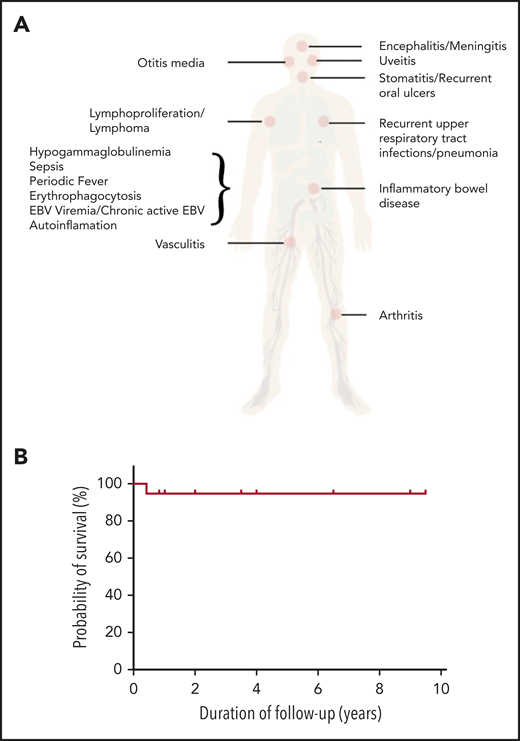
Disease spectrum and transplant outcome in patients with CD27 and CD70 deficiencies. (A) Spectrum of disease manifestation in patients with CD27 and CD70 deficiency. (B) Overall survival post-HSCT for CD27 and CD70 deficiency. The figure has been adapted from Figure 1C in the article by Ghosh et al that begins on page 2638.
Disease spectrum and transplant outcome in patients with CD27 and CD70 deficiencies. (A) Spectrum of disease manifestation in patients with CD27 and CD70 deficiency. (B) Overall survival post-HSCT for CD27 and CD70 deficiency. The figure has been adapted from Figure 1C in the article by Ghosh et al that begins on page 2638.
CD27 is expressed on a variety of T lymphocytes including naive and central memory T lymphocytes, as well as germinal center and memory B lymphocytes and plasma cells. The expression of its ligand, CD70, is restricted to activated lymphoid and myeloid cells. The CD27-CD70 axis delivers costimulatory signals to enhance T-lymphocyte activation, survival, proliferation, and differentiation; maintain antigen-specific CD4+ and CD8+ T lymphocytes; and promote memory B-lymphocyte differentiation and plasma cell survival. Thus, the CD27/CD70 axis can activate innate and adaptive immunity, has an important role in maintaining immune tolerance, and has a critical role in EBV immune surveillance.
For an increasingly recognized number of primary immune deficiencies, EBV is an important pathogen, associated with the pathogenesis of lymphoma, lymphoproliferative disorders, and/or hemophagocytic lymphohistiocytosis (HLH).2 Patients with defects in CD27 characteristically display susceptibility to EBV-associated lymphoproliferative disorders and lymphomas. Many also report fever, oral ulceration, and other infections.3 More recently, patients with defects in CD70 have been reported, with similar features including lymphoma, recurrent fevers, lymphoproliferation, persistent EBV viremia, hypogammaglobulinemia, recurrent respiratory infection, and autoimmunity.4,5 Now, Ghosh et al report the clinical features, treatment, and outcome of 49 patients (33 with CD27 defects, 16 with CD70 defects), one-half of whom were previously unreported.
A number of features are notable from this report. First, the presentation is pleiomorphic (see figure panel A). Although over 90% of patients were EBV+ at diagnosis, most presented with lymphoproliferation (71%), lymphoma (43%), and, in CD27-deficient patients only, HLH (27%). Lymphoma developed in 43% of patients before the age of 10 years. However, neither malignancy nor lymphoproliferation was inevitably the presenting feature. Autoinflammatory features were present in 43% of patients, including Behcet symptoms, uveitis, arthritis, and periodic fever. Non-EBV-related infections were also common, predominantly due to other herpesviruses, but upper and lower respiratory tract infections were also described in more than one-half of the patients. Almost 40% of patients had documented hypogammaglobulinemia. There was intrafamilial symptom variation, and some individuals were asymptomatic. In terms of treatment, given the spectrum of clinical phenotypes, a variety of approaches were documented. Patients with hypogammaglobulinemia were treated with immunoglobulin replacement and antibiotic prophylaxis, those with HLH received either the HLH-1994 or -2004 protocol, whereas those with malignancies received the appropriate disease-specific protocol. Treatment details for autoinflammatory diseases were not provided. Nineteen patients underwent allogeneic hematopoietic stem cell transplantation (HSCT), with a variety of clinical indications including recurrent infection, autoinflammation, and malignancy. A spectrum of matched and mismatched donors, stem cell sources, and conditioning regimens was used, but overall and event-free survival were 95% (see figure panel B) with a follow-up of 1 to 9.5 years. Most patients ceased immunosuppression within a year of transplant, and there was no relapse or secondary malignancies documented in those transplanted to treat lymphoma. However, in the nontransplanted group, there were 8 deaths (6 from a primary malignancy, 2 from infection) giving an overall survival of 74%.
Patients with defects in CD27 or CD70 may present to general pediatricians, pediatric pulmonologists, oncologists, rheumatologists, hematologists, or immunologists. Consequently, an awareness of this rare condition is important. EBV-associated lymphadenopathy or lymphoma are common presentations to pediatric oncologists. A debate about how many children presenting with malignancy may harbor an underlying defect in a gene associated with primary immune deficiencies is ongoing6 ; from this study, patients who have other features including a family history, or evidence of autoinflammation, recurrent infection, or hypogammaglobulinemia should be considered for further investigation. Similarly, other disease specialists to whom these patients may present should look carefully for other features in the history that may indicate this disease, particularly EBV persistence. In that respect, the authors of this study consider that EBV persistence could be used as a biomarker, although it is not particularly sensitive nor specific.
Six patients died during treatment of their first episode of malignancy. For patients with malignancy, a genetic diagnosis is important, as an early consideration of HSCT may lead to better long-term outcomes, even in those with incomplete remission, as has been recently reported for children with lymphoproliferative disorders and other immunodeficiencies.7 For other patients, the decision about transplantation can be difficult. Although the clinical course of some of the patients described in this report was encouraging following treatment of lymphoma or HLH, given the genetic and immunological deficit, there is a risk of recurrence. These patients require careful follow-up because of that risk. Given the excellent outcome of HSCT as described, it would be reasonable to consider transplantation early in the disease course if there is an appropriate donor. However, given the relatively high incidence of nonlymphoma malignancy occurring in carrier parents of both CD27 and CD70 deficiency, despite good results for other primary immune deficiencies using T-depleted grafts,8,9 the use of carrier donors is not recommended for CD27 and CD70 deficiency.
Conflict-of-interest disclosure: The author declares no competing financial interests.