Abstract
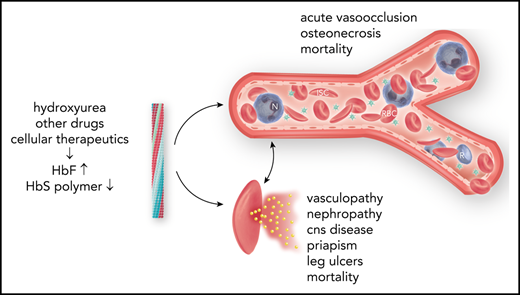
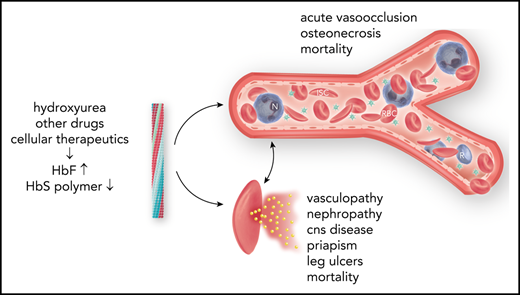
Fetal hemoglobin (HbF) can blunt the pathophysiology, temper the clinical course, and offer prospects for curative therapy of sickle cell disease. This review focuses on (1) HbF quantitative trait loci and the geography of β-globin gene haplotypes, especially those found in the Middle East; (2) how HbF might differentially impact the pathophysiology and many subphenotypes of sickle cell disease; (3) clinical implications of person-to-person variation in the distribution of HbF among HbF-containing erythrocytes; and (4) reactivation of HbF gene expression using both pharmacologic and cell-based therapeutic approaches. A confluence of detailed understanding of the molecular basis of HbF gene expression, coupled with the ability to precisely target by genomic editing most areas of the genome, is producing important preliminary therapeutic results that could provide new options for cell-based therapeutics with curative intent.
Introduction
Fetal hemoglobin (HbF; α2γ2), a minor hemoglobin of normal adults, has major clinical significance for sickle cell disease. γ-Globin is encoded in HBG2 (Gγ) and HBG1 (Aγ), nearly identical genes found in a developmentally regulated gene cluster on chromosome 11p15 (5′—ϵ—Gγ—Aγ—δ—β—3′). Sickle cell disease is caused by homozygosity for the sickle hemoglobin (HbS) gene (β7glu-val, GAG-GTG) or compound heterozygosity of the HbS gene and other HBB disorders.1 DeoxyHbS polymerization drives the disease pathophysiology. The rate and extent of polymerization is modified by the erythrocyte HbF concentration.2 Abnormal sickle erythrocytes transit small vessels slowly providing time for deoxyHbS fiber formation with resulting cell injury. HbF prolongs the time needed for HbS polymerization (delay time), allowing the escape of sickle erythrocytes from the microcirculation into larger veins and hence the lungs, where on reoxygenation, HbS polymer melts.3 HbF is variably increased in nearly all HbS homozygotes.4 The most efficient means of treatment is interrupting disease pathophysiology by preventing deoxyHbS polymerization. This can be accomplished by inducing sufficiently high levels of HbF in most sickle erythrocytes.5
Ten years have elapsed since we reviewed HbF as a modulator of sickle cell disease.4 Important developments since then include the following: (1) detailed understanding of the switch from fetal to adult hemoglobin gene expression; (2) therapeutic exploitation of this knowledge; (3) how HBB haplotypes influence HbF; (4) understanding the importance of heterogeneity of the distribution of HbF concentrations among HbF-containing erythrocytes; (5) expanded use of hydroxyurea; and (6) new targets for HbF induction. This review focuses on advances since 2011.
Quantitative trait loci associated with HbF
Inactivation and activation of genes within the β-globin gene cluster holds the keys to reactivating for therapeutic gain the usually dormant HbF genes.6-12 Transcription factor complexes interact with the locus control region (LCR) enhancers upstream of the β-globin gene cluster and with globin gene promoters, silencing embryonic and fetal genes while activating adult genes. In addition to BCL11A and ZBTB7A, the predominant HbF repressors discussed below, developmental factors including the LIN28B and IGF2BP1 RNA-binding factors and the let-7 family of microRNAs also modulate HbF gene expression. Reduction of let-7 or overexpression of its regulator LIN28B is associated with increased HBG expression.13
Three quantitative trait loci (QTL), BCL11A, MYB, and a locus in the promotor of HBG2 modify HbF gene expression. BCL11A (chr2p16), a zinc finger transcription factor, was first identified by genome-wide association studies.4,14-16 BCL11A is a repressor of HbF gene expression. Reducing bcl11a expression in transgenic sickle mice derepressed γ-globin genes.17,18 Functional variants of BCL11A are marked by single nucleotide polymorphisms (SNPs) in intron 2 that tag an erythroid-specific enhancer.19 The sentinel SNP marking this QTL is rs1427407. This enhancer consists of 3 DNase hypersensitive sites +62, +58, and +55 kb from the transcription initiation site.19 Variants associated with increased HbF are present in ∼20% of sickle cell anemia patients. BCL11A binds TGACCA motifs in the HBB gene cluster, preferentially binding to position −115 in the HBG2 and HBG1 promoters.20,21 BCL11A expression is controlled by transcriptional and/or translational mechanisms.22-24 ZBTB7A (LRF) is another zinc finger HbF gene silencer. Its inactivation in HUDEP-2 cells led to ∼50% HbF; BCL11A plus ZBTB7A knockout caused > 90% HbF.25 The relative increase in γ globin is magnified by the decrease in β globin. ZBTB7A is not polymorphic and does not account for HbF variation in patients with sickle cell anemia.26
Natural mutations and clustered regulary interspaced short palindromic repeat (CRISPR)-Cas9 disruption of the BCL11A and ZBTB7A binding sites are associated with a large effect on expression of the γ-globin genes.12,21,27 Krüppel-like factor 1 (KLF1) binds to the promoter of ZBTB7A increasing its expression; KLF1 also activates the expression of BCL11A.28,29 Reduced KLF1 expression or activity is associated with HbF derepression.
The C-T polymorphism 158 bp 5′ of HBG2, a cleavage site for Xmn1, is associated with increased HbF in the Senegal and Arab-Indian HbS gene haplotypes. CRISPR-Cas9 disruption of this site in sickle cell CD34+ cells caused ∼25% γ globin and 55 ± 5% F-cells. Disruption of BCL11A and ZBTB7A binding sites led to 30% to 40% γ globin and 75% to 80% F-cells.27 Only HBG2 expression is affected by the −158 polymorphism; both HbF genes are affected by BCL11A polymorphisms.30 HBG2 levels fall from ∼70% at birth to ∼40% 6 months postnatally, making it unsurprising that targeting the HBG2 site had a smaller effect on HbF. The transcription factors binding to the −158 HBG2 motif are uncharacterized.
Four markers representing the 3 HbF QTL, rs654815 and rs1427407 in BCL11A, rs66650371, the 3-bp deletion in MYB, and rs7482144 in the HBG2 promoter, accounted for 21.8% of HbF variability in 581 HbS homozygotes and patients with HbS-β0 thalassemia. Similar results were found in 186 patients with compound heterozygosity for HbS and HbC genes (HbSC) disease and 994 Tanzanian HbS homozygotes.31
HbF, geography, and HBB haplotypes
The HbS gene had a single origin in West Central Africa or in the Sahara during the Holocene Wet Phase between 3400 and 11 100 years ago or in agriculturists ∼22 000 years ago, which was introduced into rainforest dwellers within the last 6000 years.32,33 The earlier origin in agriculturists might have resulted from destruction of the rain forest canopy that created conditions favorable for malaria. Malaria, in turn, provided the selective pressure driving the rise of the HbS gene. From the primordial haplotype the HbS gene subsequently became associated with 5 common HBB haplotypes: Bantu (CAR or Central African Republic), Cameroon, Arab-Indian (AI), Senegal, and Benin.34 AI, Senegal, and Benin haplotypes are derived from the CAR and Cameroon haplotypes.32 Each haplotype is associated with a distinctive mean HbF level, albeit with wide dispersion, making ascertainment of haplotype in individuals of little prognostic value.
Adult HbS homozygotes with the AI haplotype have a mean baseline HbF of 17%35 ; Senegal haplotype patients have HbF of ∼10%. Among Benin, Bantu, and Cameroon haplotype patients, the HbF level of individuals of African and Arab descent appear to differ. Benin haplotype patients of African descent have HbF of ∼6%; Saudi Arab patients with the Benin haplotype have HbF of ∼11%.36 Saudi patients with Bantu and Cameroon haplotypes have HbF of ∼8% compared with ∼5% in their African counterparts.35 Children with the AI haplotype have HbF of ∼30%.37 As HbF levels decline, AI haplotype adults have more numerous symptoms, and their disease begins to resemble that of African patients, but with a lower incidence of stroke, no leg ulcers, and >70% prevalence of splenomegaly.37,38 HbF differences among haplotypes were not associated with BCL11A polymorphisms.39 With the possible exception of the AI and Senegal haplotypes, the genetic basis of the variance of HbF levels among HbS gene haplotypes remains undefined.
The AI haplotype in Saudi patients is associated with a unique pattern of SNPs in putative cis-acting globin regulatory elements and is linked to a novel variant in ANTXR1.40,41
A 3-SNP subhaplotype of the AI haplotype defined by 2 polymorphisms in the LCR and rs7482144 was unique to Saudi Arabs. Homozygosity for minor alleles at rs16912979, rs7119428, and rs7482144 (T/A/T) were hypothesized, but not mechanistically verified, to represent a cis-acting domain modulating HBG2 expression by facilitating LCR contacts with the HBG2 promoter.40 The Senegal haplotype, with intermediate HbF levels, contains rs7482144; Benin, Bantu, and Cameroon haplotypes lacking rs7482144 have the lowest HbF.
BCL11A and MYB accounted for only 8.8% of HbF variance in the AI haplotype population, suggesting that other trans-acting elements might influence their high HbF. An intronic SNP (rs35685045) in ANTXR1 (TEM8; chr2p13) was associated with HbF in 4 cohorts of adult AI haplotype patients.41,42 This variant explained ∼10% of HbF variability compared with ∼8% for BCL11A. Like BCL11A and MYB, ANTXR1 appeared to be a repressor of HbF. ANTXR1, a type 1 transmembrane protein, is expressed at high levels in bone marrow. It remains possible that ANTXR1 could be acting through BCL11A despite their additive and independent effects on HbF and absence of linkage disequilibrium between ANTXR1 and BCL11A SNPs.
F-cells: heterogeneity of HbF concentrations
Erythrocytes with detectable HbF are called F-cells. These cells arise stochastically from erythroid precursors that can give rise to both HbF-containing and non–HbF-containing erythrocytes. BCL11A and ZBTB7A are not differentially expressed in F-erythroblasts compared with non–HbF-expressing erythroid precursors. F-cells do not resemble fetal erythrocytes save for their high levels of HbF.43 Patients with sickle cell anemia have 2% to 80% F-cells, which survive longer than non-HbF cells. Improved F-cell survival is likely a result of HbF protecting the cell from HbS polymer-induced damage.44-47 This protection provided by HbF can be nearly absolute or only partial. Treatment with hydroxyurea increases the number of F-cells and the concentration of HbF/F-cell.
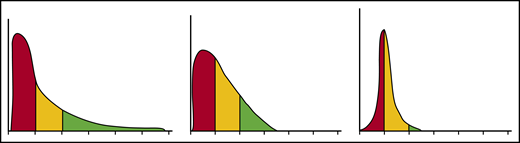
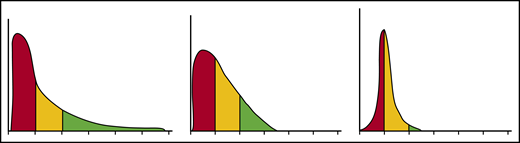
Each erythrocyte contains ∼30 pg hemoglobin. The threshold for F-cell detection by fluorescent activated cell sorting (FACS) using an antibody reactive with HbF is ∼6 pg of HbF/F-cell. When HbF/F-cell is 9 to 12 pg, calculations suggested that deoxyHbS polymerization should be prevented at physiologic venous and capillary O2 saturations of 40% to 70%, affording the sickle erythrocyte nearly total protection from HbS polymer-induced damage, although recent studies call into question this observation.3,48,49 In compound heterozygotes for HbS and the common gene deletions HPFH-1 and HPFH-2 that cause hereditary persistence of HbF (HPFH), total HbF level is ∼30%. HbF is distributed nearly evenly, or pancellularly, in HbS-HPFH erythrocytes, endowing each cell with ∼10 pg HbF; HbS polymer is not present in these erythrocytes. Individuals with this genotype do not have hemolytic anemia and are usually asymptomatic.50 In sickle cell anemia, HbS polymer is present at 70% O2 saturation, and the polymer fraction rises steeply as O2 saturation falls.51 Patients with untreated sickle cell anemia, sickle cell anemia treated with hydroxyurea or HbS-δβ thalassemia, who can have total HbF levels similar to those found in HbS-HPFH, are usually symptomatic; all have hemolytic anemia. The contrast between the clinical and hematologic features of HbS-HPFH, and these conditions suggest that HbF level or F-cell percentage are poor predictors of disease severity in an individual because neither accounts for the variable distribution of HbF levels among F-cells. We mathematically modeled possible distributions of HbF/F-cell in patients with HbF levels of 5%, 10%, 20%, and 30%, reflecting, respectively, the mean levels of HbF in adult African-origin patients with the Benin/Bantu/Cameroon and Senegal haplotypes and adult and pediatric patients with the AI haplotype.52 It is nearly impossible to have a clinically important number of fully protected F-cells when HbF is 5%; no more than 15% of cells can be protected when HbF is 10%.52 This is consistent with disease severity in most patients with these HbF levels and individuals with Bantu/Benin/Cameroon haplotypes. Consistent with the phenotype of most patients with natural or therapy-induced HbF levels of 20%, it is possible for nearly 25% of F-cells to have polymer-inhibiting HbF levels. When HbF is near 30%, disease can be relatively benign and 70% of cells can be protected.52-54 (Figure 1). The number of F-cells with polymer-inhibiting concentrations of HbF is likely to be a more important determinant of the features of sickle cell anemia than the total number of F-cells or the concentration of HbF in the hemolysate. A prospective study where the distribution of HbF concentrations in F-cells can be rapidly and repeatedly measured and that controls for other hematologic variables like α thalassemia is needed to confirm this hypothesis.
HbF distribution in sickle erythrocytes from 3 patients with 20% HbF. An example of 3 possible distributions of HbF/F-cell in 1000 cells from 3 individuals with mean HbF levels of 20%. The y axis represents numbers of cells, and the x axis represents HbF concentration in 5-pg increments. In red are cells likely to be least protected from HbS polymer damage. These cells have HbF < 6 pg and are not visible by FACS. In yellow are cells visible by FACS but not fully protected from HbS polymerization. They have HbF concentrations of 6 to 10 pg. In green, are cells with HbF concentrations >10 pg. These cells should be fully protected from HbS polymerization. Many other distributions are possible with the same mean HbF accounting for the varied phenotypes associated with the same HbF concentration. Data are derived from Steinberg et al.52
HbF distribution in sickle erythrocytes from 3 patients with 20% HbF. An example of 3 possible distributions of HbF/F-cell in 1000 cells from 3 individuals with mean HbF levels of 20%. The y axis represents numbers of cells, and the x axis represents HbF concentration in 5-pg increments. In red are cells likely to be least protected from HbS polymer damage. These cells have HbF < 6 pg and are not visible by FACS. In yellow are cells visible by FACS but not fully protected from HbS polymerization. They have HbF concentrations of 6 to 10 pg. In green, are cells with HbF concentrations >10 pg. These cells should be fully protected from HbS polymerization. Many other distributions are possible with the same mean HbF accounting for the varied phenotypes associated with the same HbF concentration. Data are derived from Steinberg et al.52
HbF effects on clinical and hematologic phenotypes
High HbF is strongly associated with a reduced rate of acute painful episodes, fewer leg ulcers, and longevity.55 Any protection afforded by HbF against the development of priapism, renal functional impairment, cerebrovascular disease, and perhaps sickle vasculopathy is questionable. HbF is also associated with higher O2 saturations.56 Unequal protection by HbF across the gamut of sickle cell disease complications might be partially explained by differences in the pathophysiologic roots of these complications.57,58 Acute painful episodes and acute chest syndrome are a result of sickle vasoocclusion; HbF has the most noticeable beneficial effect on these subphenotypes. Priapism, nephropathy, and sickle vasculopathy are associated with hemolysis. In these complications HbF has a less obvious beneficial effect, perhaps because only a small number of lysed sickle erythrocytes are needed to trigger these subphenotypes.59
HbF and survival
In 3764 patients in the pre-hydroxyurea era, HbF > 8.6% was associated with improved survival.60 Recent studies have documented the importance of hydroxyurea-induced increases in HbF. A meta-analysis of 7 studies with 2477 hydroxyurea era cases showed that HbF was associated with reduced mortality (hazard ratio [HR]: 0.97; 95% confidence interval [CI]: 0.94-1.0).61 Increased HbF associated with taking 15 to 35 mg/kg hydroxyurea improved survival in 393 HbS homozygotes (HR: 0.36; 95% CI: 0.17-0.73).62 Using the PedsQL Sickle Cell Disease Module, health-related quality of life in 123 Omani children with sickle cell disease was predicted by HbF independent of other measured elements (95% CI: 0.15-1.12).63
To identify prognostic factors for clinical complications, 57 infants were studied at 4.4 ± 1 months of age and followed for ∼19 months. HbF, which fell from 42% to 21% during the observation period, was the strongest prognostic factor for disease complications.64 Of 230 children treated with hydroxyurea with 510 patient-years of follow-up, patients with HbF less than 20% had twice the risk of hospitalization for pain and acute chest syndrome and 2 times the risk of fever as patients with >20% HbF.65
HbF and cerebrovascular disease
Pre-hydroxyurea era studies of patients with a mean age of 14.1 ± 12.7 years suggested that neither stroke nor silent cerebrovascular disease was associated with HbF.66,67 More recent work suggested a protective effect of HbF. Children aged 12.1 ± 2.6 years with the highest HbF were protected from silent infarctions.68 Of 375 children with sickle cell anemia or HbS-β0 thalassemia followed in a longitudinal prospective study, 59 had cerebrovascular disease or a stroke at a mean age of 4.4 years; HbF was protective (HR: 0.68; 95% CI: 0.48-0.96).69 Eighty-three neurologically intact adults with sickle cell anemia had silent white matter changes that were associated with low HbF.70 In early childhood, high HbF is likely to be associated with more F-cells containing protective HbF levels. As HbF falls and fewer F-cells are protected from HbS polymer damage, the propensity for vascular occlusion and intravascular hemolysis increases with the release of heme products into the circulation that scavenge nitric oxide causing endothelial damage and vasculopathy.57-59
HbF and malaria
HbF did not provide protection from malaria in African (HbF, 5.6%) or Indian (HbF, 18.2%) sickle cell anemia patients.71,72 Nevertheless, an open-label trial in 606 African children aged 1 to 10 years treated with hydroxyurea found that HbF increased from 10.9% to 23.4%. This was associated with a decrease in the rate of malaria infection by more than 60%.73 Malarial parasites grow poorly in HbF-containing erythrocytes. The stability of the HbF compared with the HbA tetramer might account for part of this effect.74
Therapeutic induction of HbF
Hydroxyurea
Hydroxyurea is recommended for nearly all patients with sickle cell anemia or HbS-β0 thalassemia starting in the first year of life; it is also is used in symptomatic HbSC disease and HbS-β+ thalassemia. Fourteen children with HbSC disease, mean age 11 years, were given hydroxyurea at the maximum tolerated dose. HbF increased from 1.7 ± 1.9% to 6.3 ± 6.7%.75 After 12 months, HbF increased from ∼2.8% to 5.5% in 133 hydroxyurea-treated HbSC disease patients.76 In 39 Italian patients with HbS-β+ thalassemia receiving more than 15 mg/kg of hydroxyurea, HbF increased from 4.7% to 10.9%.77 Greek adults with HbS-β+ thalassemia treated with hydroxyurea showed a HbF increase from a median of ∼6.8% to a median of ∼20%.78 Hydroxyurea is also used effectively in low-resource countries.73,79,80
By inducing increased levels of HbF through mechanisms that include suppressing erythropoiesis, hydroxyurea was first associated with reduction of morbidity and mortality in adults.81,82 Hydroxyurea treatment was associated with progressive increases in the polymerization delay time, coincident with the increase of F-cells.83 Some benefits of hydroxyurea might also be unrelated to HbF induction.83
HbF induction by hydroxyurea depends in part on a proliferating erythroid bone marrow, which is most vigorous in childhood. With time, intramedullary sickling and marrow infarction reduce the erythroid capacity of the marrow.84,85 Following 2 years of hydroxyurea in adults with baseline HbF of ∼5%, the top quartile of HbF responders had ∼12% HbF.86 In contrast, all children seem to have a robust HbF response to hydroxyurea that retards the natural age-related fall in HbF.87 At a fixed dose of 20 mg/kg, after at least 18 months of observation, the percent decrease in HbF in hydroxyurea-treated patients was 13% compared with 37% in placebo-treated controls. When hydroxyurea was started at 9 to 18 months of age, HbF remained at its baseline level of 25%. Nonetheless, even in treated patients, HbF levels might decline with age. Following 2 years of treating children <2 years old at a dose of 20 mg/kg, HbF was 19.7%; after 15 years, HbF fell to 15.1%. Therefore, even when started early in life, the HbF response to hydroxyurea might wane.88 Hydroxyurea started at ∼11 months at a dose of ∼27 mg/kg was associated with HbF levels of 33.3 ± 9.1%; 33% of patients had HbF > 40% with a hemoglobin concentration of 10.1 ± 1.3 g/dL; acute events were markedly reduced; and toxicity was minimal.89 This dosing approach is setting a new standard for using hydroxyurea with a profound effect on the disease in childhood. Dose escalation was also superior to fixed dosing when studied in Ugandan children.80 The ultimate safety and efficacy of this approach will require long-term follow-up.
Hydroxyurea was noninferior to transfusion for reducing transcranial Doppler flow velocities in selected children at high risk for stroke. Whether this observation was a direct benefit of HbF, a secondary benefit of the higher hemoglobin concentration caused by increased HbF, or a combination of these is uncertain.90
Novel agents and targets
Other drugs that act epigenetically or with novel mechanisms of action have increased HbF levels in preclinical and early-phase clinical trials; none are approved for treatment. The current status of these agents and new targets for HbF induction is summarized in Table 1.
Cell-based HbF-inducing therapeutics
Cure of sickle cell anemias is possible if enough HbF can be induced in enough sickle erythrocytes to thwart their premature destruction and inhibit sickle vasoocclusion. This has not yet been achieved with drug therapy but might be possible with cell-based therapeutics. Cell-mediated therapy either adds a β-globin gene with a HbF-like substitution or reverses the silencing of the HbF genes by curbing HbF repressors or disrupting their binding motifs in γ-globin gene promoters.
Lentiviral-mediated gene addition.
More than 40 years ago, Nagel et al114 showed that the glutamine (Q) at position 87 in the γ-globin chain was 1 of 2 residues accounting for the inhibitory effect of HbF on HbS polymerization. Replacing β87 threonine (T) with Q (HbAT87Q) and incorporating this gene into a lentiviral vector that was transferred into hematopoietic stem cells corrected the main features of sickle cell disease in transgenic mice.115 In a phase 1/2 study, a similar HbAT87Q-lentiviral construct was introduced into autologous CD34+ stem and progenitor cells that were infused into myeloablated patients with severe forms β thalassemia. The requirement for chronic transfusion was reduced or eliminated in all patients.116 This same approach was used in a patient with sickle cell anemia. About 1 copy of this vector was present in each cell. After 15 months, total hemoglobin level was 11 to 12 g/dL; HbAT87Q formed about half of the total hemoglobin; and the patient was asymptomatic.117 Using a modified manufacturing process, 13 patients had plerixafor mobilized CD34+ cells transfected with the HbAT87Q-containing vector and were followed for 3 to 15 months. The vector copy number was 3.8, HbAT87Q was nearly 50% of total hemoglobin, and hemoglobin levels were 10.2 to 15 g/dL; patients became asymptomatic.118
Lentiviral-mediated short hairpin RNA knockdown of BCL11A.
A lentiviral vector containing a short hairpin RNA (shRNA) embedded in a microRNA scaffold was used to allow erythroid-specific knockdown of BCL11A (shRNAmiR) after insertion into autologous CD34+ cells.119 Preclinical testing in sickle CD34+ cells transduced with this shRNAmiR achieved a vector copy > 5 and gene marking of > 80%, resulting in a three- to fivefold induction of HbF. Growth, differentiation, and engraftment of gene-modified cells in vitro or in vivo were unaffected. Three patients treated with this vector and followed for 7 to 15 months had HbF of 23.7% to 40.7% and F-cells of 67.7% to 76.4%; total hemoglobin was >11 g/dL; and acute vasoocclusive events stopped (NCT03282656).120
Targeting HbF repressors by genomic editing.
The toolkit for genome editing is rapidly expanding. CRISPR-Cas dominates the field because of the ease of using guide RNAs directing the CRISPR complex to specific DNA sequences and the increasing ability to target most sites in the genome.121,122 In addition to the ability to create small deletions and insertions using traditional CRISPR-Cas9 technology, it is also possible to achieve some single base edits by nicking but not cleaving DNA. The favored repair mechanism for double-strand DNA breaks in human cells is nonhomologous end joining that leads to disruption of normal sequence in the targeted area, introducing small insertions or deletions. In the context of sickle cell disease treatment discussed below, CRISPR-Cas9 disrupts specific enhancer motifs or binding sites for HbF repressor proteins. Correcting the HbS mutation itself requires a slightly different approach relying on homology-directed repair, which as currently used, is a less efficient reparative pathway.
Several approaches to reducing the expression of HbF suppressors in CD34+ cells have entered clinical trials, including the first use of CRISPR-Cas9 in humans (Figure 2).123-125 They include disrupting the erythroid enhancer of BCL11A or the binding sites for BCL11A in the γ-globin gene promoters.126 Clinical trials are targeting BCL11A with CRISPR/Cas and zinc finger nucleases (NCT03745287, NCT03655678, and NCT03653247). In a phase 1/2 study, after stem cell mobilization with plerixafor and myeloablative conditioning with busulfan, a single infusion of autologous CD34+ cells that were edited with CRISPR-Cas9 using a guide RNA specific for the erythroid enhancer region of BCL11A was given to a patient with sickle cell anemia. Engraftment occurred after 30 days. The baseline HbF of 9.1% increased to 25.9% after 2 months and appeared to stabilize between 46% and 48% when tested at 4 through 9 months; F-cells increased from 33.9% to 99.7%; total hemoglobin stabilized at 11 to 12 g/dL; and vaso-occlusive episodes stopped.125 Two patients with β thalassemia treated similarly achieved HbF >97% of the hemolysate with a hemoglobin concentration at 15 months of 14 g/dL.125
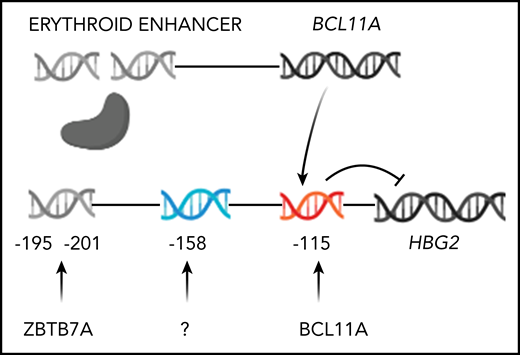
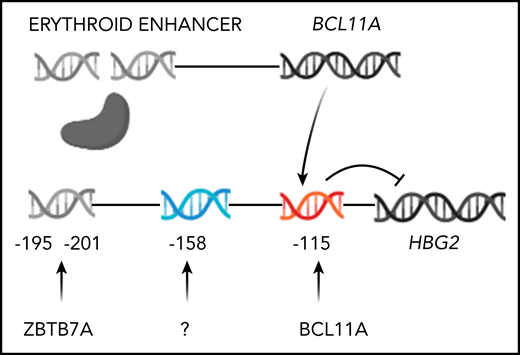
Genome editing to increase HbF production. Only HBG2 and its upstream binding sites for BCL11A and ZBTB7A are depicted. The expression of BCL11A, 1 of 2 major repressors of HbF gene expression, is controlled by an erythroid-specific enhancer. BCL11A binds to a TGACCA motif centered at position −115 in the promoters of both HBG2 and HBG1. ZBTB7A, the other major HbF gene repressor, not shown, binds at positions −195 to −197 and −201 to −202 upstream of both γ-globin genes. A still unknown transcription factor(s) is likely to bind the −158 site. The −158 polymorphism is found only in HBG2. CRISPR-Cas9 editing of either the BCL11A erythroid-specific enhancer, shown as a double-strand break, or its binding sites in the HbF gene promoters, shown before editing, reverses the repression of these genes increasing HbF.
Genome editing to increase HbF production. Only HBG2 and its upstream binding sites for BCL11A and ZBTB7A are depicted. The expression of BCL11A, 1 of 2 major repressors of HbF gene expression, is controlled by an erythroid-specific enhancer. BCL11A binds to a TGACCA motif centered at position −115 in the promoters of both HBG2 and HBG1. ZBTB7A, the other major HbF gene repressor, not shown, binds at positions −195 to −197 and −201 to −202 upstream of both γ-globin genes. A still unknown transcription factor(s) is likely to bind the −158 site. The −158 polymorphism is found only in HBG2. CRISPR-Cas9 editing of either the BCL11A erythroid-specific enhancer, shown as a double-strand break, or its binding sites in the HbF gene promoters, shown before editing, reverses the repression of these genes increasing HbF.
All cell-based modalities had similar results with close to 50% HbF or HbAT87Q. Many of these studies had follow-up of more than 1 year, with near normalization of the hematologic findings. This suggests that stress erythropoiesis contributes minimally, if at all, to high HbF. Genomic editing and shRNA-mediated knockdown of BCL11A should erase lingering doubts that the clinical and hematologic phenotypes of sickle cell anemia can be reversed by high HbF levels in most sickle erythrocytes. If these genetic modalities are safe, effective, and similarly priced, how might one choose between them and allogeneic stem cell transplantation? Gene therapy does not require the immunosuppression. Nevertheless, experience with allogeneic transplantation is far greater, and matched-sibling transplants, especially in young children, provide excellent results.127 Gene therapy will also have to compete with the improving prospects for haploidentical allogeneic transplantation that could extend the proven effectiveness of allogeneic transplantation.128 Myeloablative conditioning is required for all cell-based therapeutics but has been associated with myelodysplasia; other forms of conditioning might be available in the future.129,130 Genomic editing does not use lentiviral transduction. Off-target consequences of genome editing, adverse effects of semirandom integration of lentivirus, and the sustainability of therapeutic effects need long-term study.
Conclusion
Cell-based therapeutics are developing rapidly. Perhaps in 10 years, when HbF is next reviewed, the current elegant efforts will be seen as crude approaches displaced by in vivo gene therapies with intravenous introduction of a therapeutic vector that homes to the targeted stem cell. Even better would be an affordable oral drug, or combination of drugs, that pancellularly raises HbF levels or otherwise prevents HbS polymerization.
Acknowledgments
This study was supported in part by the National Heart, Lung, and Blood Institute, National Institutes of Health grants R01 HL068970 and R01 HL133350 and the Office of Collaboration and Knowledge Exchange, University of Dammam, Saudi Arabia grant SP 11/2011.
Authorship
Contribution: M.H.S. wrote the paper.
Conflict-of-interest disclosure: M.H.S. sits on the advisor board for Vertex/CRISPR and Fulcrum Therapeutics and on the data monitoring committee for Imara.
Correspondence: Martin H Steinberg, Boston Medical Center, Room 211, Evans Building, 72 E Concord St, Boston, MA 02118; e-mail: mhsteinb@bu.edu.