Background: Insufficient thymic function of allogeneic hematopoietic stem cell transplantation (allo-HSCT) receptors results in continuous production of alloreactive T cells, which leads to the development of graft-versus-host disease (GVHD), especially chronic GVHD (cGVHD). We have previously found that patients with acute GVHD (aGVHD) treated with mesenchymal stem cells (MSCs) have increased thymic output and decreased incidence of cGVHD, thus hypothesized that MSCs may reduce the incidence of cGVHD by remodeling the thymus. Chemokine receptor 9 (CCR9), the receptor that specifically guides migration of T-lineage precursors into thymus, is also expressed on MSCs, and thus may be a key factor mediating MSCs homing to the thymus. This in turn allows MSCs to reduce GVHD by repairing thymus tissue structure and saving thymus function.
Methods: We carried out studies in a murine GVHD model of fully MHC-mismatched myeloablative bone marrow transplantation (C57BL/6 to BALB/c), a model that can observe the prolongation of aGVHD to cGVHD. We randomly divided GVHD mice into four groups, including three MSCs treated groups and one untreated group. CCR9 over-expressed (MSC/CCR9+), knocked-down (MSC/CCR9-) and empty-load MSCs (MSC/Control) were generated and administrated intravenously at dose of 5 × 105 cells/infusion at 7th and 21th day post HCT to the treated groups respectively to compare their thymic homing ability, and therapeutic effects of GVHD with the untreated group. Clinical scores were recorded once every five days to evaluate GVHD symptoms. Mice of MSCs treated groups and the untreated group were sacrificed at 30d, 45d and 60d after HCT. Thymuses of each group were collected and assessed for size and weight before being manufactured into frozen sections or thymic single-cell suspension. We then analyzed the number and distribution of MSCs in the thymus of the treated groups to assess the role of CCR9 in thymic homing, and analyzed the expression of thymic T cells subsets (CD4+CD8-, CD4-CD8+, CD4+CD8+ T, CD4+CD25+Foxp3+Tregs), thymic epithelial cells (TECs) substes (CD45-CD326+Ly51+ cortical TECs and CD45-CD326+UEA-1+ medullary TECs) and the level of T cell receptor rearrangement excision circles (TRECs) in thymus among the four groups to evaluate the repair effect of MSCs for thymus. Radiation-pretreated murine TECs were cultured alone or co-cultured with murine MSCs in vitro to assess the effect of MSCs on damaged TECs.
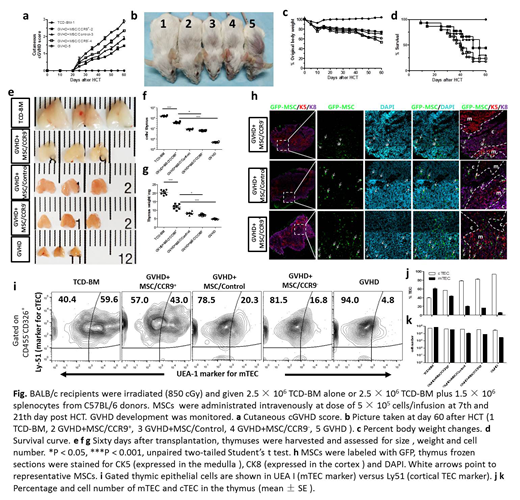
Results: The infusion of MSC/CCR9+ potently alleviated the clinical signs of GVHD and prolonged the survival of GVHD mice (P<0.05 versus MSC/CCR9- and untreated group). Significant increases in thymus size and weight were observed in the MSC/CCR9+ group, as well as the number of total thymocytes and the more organized cortical medullary structure compared to the other groups. MSCs enter the thymus from the microvascular region at the cortex-medium junction. MSC/CCR9+ were found to appear in the cortex-medium junction of thymus in a greater amount 24 hours after the first infusion, then distribute throughout the whole thymus and relocate in proximity with TECs 48 hours thereafter. MSC/Control could be observed in the cortical and cortex-medium junction, whereas MSC/CCR9- was observed only in the cortex-medium junction with a small amount of distribution. Immunofluorescence of thymus frozen sections showed that, compared with other groups, TECs had decreased apoptosis and significantly increased proliferation and maturation levels in MSC/CCR9+ group, indicating MSCs potently repaired injured TECs and promoted their proliferation and maturation. The number of TECs and its proportion of thymus stroma were significantly improved, including cortical TEC and medullary TECs. As for thymocyte, MSC/CCR9+ infusion significantly increased the number and proportion of CD4+CD8+T cells and Tregs, which were reported deficiency in GVHD thymus. Furthermore, MSC/CCR9+ administration resulted in a remarkable increase in the levels of TRECs in the thymocyte at 45d and 60d after HCT (P<0.05 versus MSC/CCR9- and untreated group). In vitro study showed co-cultured TECs had a decreased apoptosis and increased proliferation compared to TECs cultured alone.
Conclusion: This study demonstrates that CCR9 plays an important role in guiding migration of MSCs to thymus and thus highly intensify their issue repair and immunomodulatory effect to rescue thymus function in GVHD model.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.