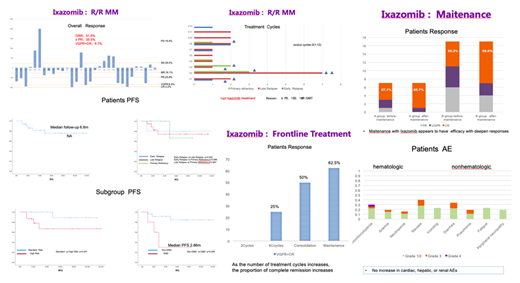
OBJECTIVE:To review the role of ixazomib in the treatment of MM from a single center in China, focusing on the efficacy and safety of this oral PI at all stages of disease since ixazomib was approved by CFDA in 2018.METHODS:The data of real-world outcomes were collected from of the patients with high-risk multiple myeloma diagnosed in the blood diseases hospital,CAMS&PUMC from July 2018 to June 2019 when the patients received the first Ixazomib cycle. The patients were divided into three groups, which were salvage therapy of R/RMM, maintenance and frontline therapy of NDMM respectively.RESULTS:The first group was Ixa-based salvage treatment in R/RMM.31 patients were included and median age was 62years(47-71).The proportion of patients with cytogenetic high risk was 50%(One patient was unable to stratify risk). Extramedullary invasion was very high 40% in early relapse. Half of them received more than 2 lines of treatment, and about 10% received 4 or more lines of prior treatment. ORR was 51.6%; ≥ PR 35.5%; VGPR+CR9.7%.The median ixazomib therapy cycles were 3(1-12). 5 patients entered maintenance therapy for more than eight cycles.In all 8 patients who interruptedIxazomib treatment,six were due to disease progression,one was SD,and the remaining one will receive CART therapy.The median follow-up was 6.8 months, and the median PFS was not yet achieved.The survival curves of high-risk group and standard-risk group were obviously separated, but there was no statistical difference.However, the prognosis of extramedullary invasion group was very poor. PFS was significantly shorter than that of control group after treatment with Ixazomib(2.86m vs NA).The second group was Ixa-based maintenance therapy of NDMM. Seven patients received maintenance therapy after ASCT, and 20 transplant-ineligible(TIE) patients entered maintenance therapy.Maitenance with Ixazomib appears to have efficacy with deepen responses,the transplantation group(57.1%CR before vs 85.7%after) similar to the non-transplantation group(35.3%before vs 58.8%after).All the patients did not interrupt Ixazomib treatment.Only 1 patient(3.7%) had grade 4 adverse events, which was thrombocytopenia.The median ixazomib therapy cycles were 4(1-11).The third group was Ixa-based frontline therapy. The median age of 24 patients was 66y (42-79), CA High Risk was 25% (6/24), R-ISS III Stage was 12.5%(3/24), and no patients with EMD.The total of 9 patients were suitable for transplantation. Among them, 5 patients completed stem cell collection, 3 patients completed autotransplantation, 1 patient waited for transplantation, and 1 patient entered the continuous treatment group because of poor mobilization.The other 4 patients were still in induction stage. There were 15 patients in the TIE group and one patient dropped out of the group because of disease progression.The remaining 23 patients achieved a overall response rate of 78.3%. Nearly half of the patients achieved deep remission(VGPR+CR 43.5%).Posttransplantation patients could achieve 100% deep remission rate.As the number of treatment cycles increased, the proportion of complete remission increased in TIE patients.CONCLUSION:Ixazomib as induction/consolidation after ASCT, followed by maintenance, is well tolerated, convenient, and effective. Ixazomib is an important option for high-risk patients with real-world experience.KEYWORDS:Real-world, Ixazomib, multiple myeloma
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.