Background: Bruton tyrosine kinase (BTK) plays a critical role in B-cell receptor signaling, which mediates B-cell proliferation, migration, and adhesion. First generation BTK inhibitor ibrutinib has limited activity as monotherapy in R/R FL (Gopal et al. J Clin Oncol 2018). Zanubrutinib (BGB-3111) is an investigational, next-generation BTK inhibitor that was designed to maximize BTK occupancy and minimize off-target inhibition of TEC- and EGFR-family kinases. Increased specificity may minimize toxicities reported with ibrutinib potentially due to off-target inhibition such as diarrhea, thrombocytopenia, bleeding, atrial fibrillation, rash, and fatigue (Coutre et al. Blood Advances 2019). In non-clinical studies, zanubrutinib has been shown to be highly potent, selective, bioavailable, and irreversible, with potentially advantageous pharmacokinetic (PK) and pharmacodynamic properties. Complete and sustained BTK occupancy has been observed with zanubrutinib treatment in both peripheral blood mononuclear cells and in lymph nodes (Tam et al. Blood 2019). Based on drug-drug interaction studies and population PK analyses (internal data), zanubrutinib may also be co-administered with strong or moderate CYP3A inhibitors at a reduced dose, proton pump inhibitors, vitamin K antagonists, as well as direct oral anticoagulants. In preclinical studies, zanubrutinib had minimal inhibitory effects against ITK and did not inhibit ITK-mediated anti-CD20-induced antibody-dependent cell-mediated cytotoxicity (Li et al. Cancer Res 2015). Zanubrutinib does not prolong the QT interval. Pooled clinical data from 6 zanubrutinib monotherapy trials including 682 patients (pts) with either non-Hodgkin lymphoma (NHL), Waldenström macroglobulinemia, or chronic lymphocytic leukemia suggests that zanubrutinib was generally well tolerated amongst pts with B cell malignancies (Tam et al. EHA 2019). This data further showed that some toxicities often associated with BTK inhibitors were infrequent with zanubrutinib, including 1.9% atrial fibrillation/flutter (0.6% grade ≥3), 2.5% major hemorrhage (2.1% grade ≥3), 10.9% fatigue (0.7% grade ≥3), 18.0% rash (0.1% grade ≥3), 18.3% thrombocytopenia (6.6% grade ≥3), and 19.4% diarrhea (0.9% grade ≥3). Early clinical data from a phase 1b dose-escalation study including 36 pts with R/R FL (median 2 [range, 1-9] prior lines of therapy) treated with the combination of zanubrutinib with anti-CD20 antibody obinutuzumab reported an overall response rate (ORR) of 72.2% including complete response in 14 pts (38.9%); median progression-free survival was 24.9 mo (Tam et al. ICML 2019).
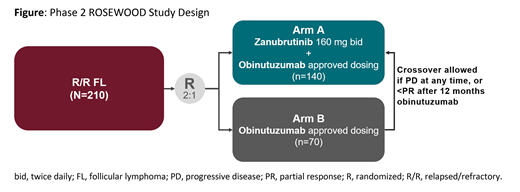
Study Design and Methods: This ongoing phase 2, global, randomized, open-label, active-controlled study (ROSEWOOD; NCT03332017) is examining zanubrutinib + obinutuzumab vs. obinutuzumab monotherapy in pts with R/R FL who have received ≥2 prior lines of therapy (Figure). Eligible pts must have histologically-confirmed grade 1-3a B-cell FL and measurable disease, and have received prior anti-CD20 antibody and alkylator-based combination therapy. Pts are randomized 2:1 to receive oral zanubrutinib 160 mg twice daily + obinutuzumab or obinutuzumab alone (both arms in 28-day cycles, at 1000 mg IV on days 1, 8, and 15 of cycle 1; day 1 of cycles 2-6; and then once every 8 weeks) until progressive disease (PD), toxicity or a maximum of 30 mo of obinutuzumab. Pts receiving zanubrutinib should remain on study treatment until PD. Randomization is stratified by prior therapies (2-3 vs >3) and rituximab-refractory status. Disease response is assessed per the 2014 Lugano Classification for NHL. The primary endpoint is ORR by independent review committee (IRC). Response rates will be compared between groups in an intent-to-treat analysis. Key secondary endpoints include ORR by investigator assessment, rate of complete response or complete metabolic response, time to and duration of response, progression-free survival (all IRC and investigator assessments), overall survival, and safety. At the investigator's discretion, pts in the obinutuzumab arm can cross over to the combination arm if they have PD at any time or less than partial response after 12 mo. Recruitment is ongoing.
Fowler:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Trotman:Celgene: Research Funding; BeiGene: Research Funding; Pharmacyclics: Research Funding; Roche: Research Funding; Janssen: Research Funding. Auer:Hartley Taylor: Honoraria; Janssen: Honoraria, Other: personal fees, Research Funding; Bristol-Myers Squibb: Other: personal fees; Celgene: Other: personal fees. Flowers:TG Therapeutics: Research Funding; National Cancer Institute: Research Funding; Spectrum: Consultancy; V Foundation: Research Funding; BeiGene: Consultancy, Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Karyopharm: Consultancy; Acerta: Research Funding; Gilead: Consultancy, Research Funding; Denovo Biopharma: Consultancy; AstraZeneca: Consultancy; Pharmacyclics/Janssen: Consultancy, Research Funding; Millenium/Takeda: Research Funding; Bayer: Consultancy; Burroughs Wellcome Fund: Research Funding; AbbVie: Consultancy, Research Funding; Optimum Rx: Consultancy; Eastern Cooperative Oncology Group: Research Funding. Reed:BeiGene: Employment, Equity Ownership, Other: Travel & Accommodations. Ivanova:BeiGene: Employment. Huang:BeiGene: Employment, Equity Ownership. Zinzani:TG Therapeutics: Honoraria, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Immune Design: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Zanubrutinib is an investigational agent and has not yet been approved in the US
Author notes
Asterisk with author names denotes non-ASH members.