Introduction
Polypharmacy, commonly defined as taking ≥5 prescribed medications, is a major issue in adults, especially those ≥60 years of age. Drug-drug interactions can significantly increase toxicities while undergoing chemotherapy, and may affect OS in AML. Polypharmacy can also increase treatment costs. We aimed to evaluate the effects of polypharmacy on OS in AML.
Methods
We included adult AML patients diagnosed from 2000-2016 at University of Nebraska Medical Center. Based on the number of prescribed medications at the time of AML diagnosis, patients were divided into 2 groups: patients with (≥5 medications) versus without polypharmacy (<5 medications). Fisher's exact test was used to identify the association of polypharmacy with baseline characteristics. OS, defined as the time from diagnosis of AML to death from any cause, was determined by the Kaplan-Meier method, and comparisons of survival curves was done using the log-rank test. Cox proportional hazard model was used to determine the effect of polypharmacy on OS adjusting for other covariates for the entire cohort, and separately for <60 years and ≥60 years old.
Results
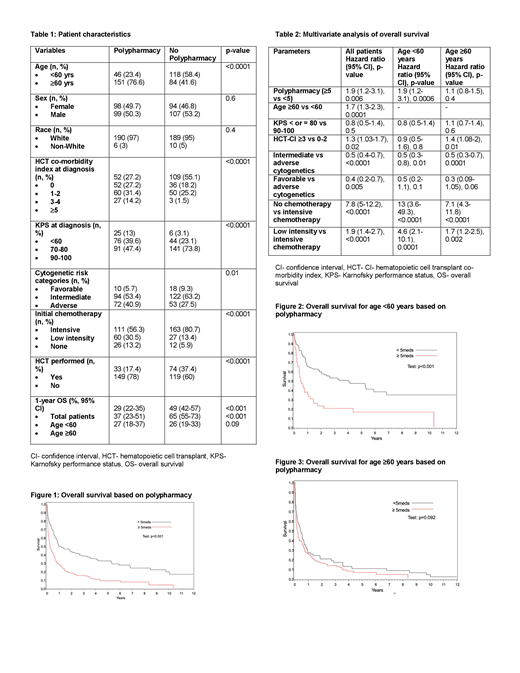
We included a total of 399 patients in the study- 59% were ≥60 years, 52% were male, 96% were white, and 34% had adverse cytogenetics (Table 1). Median number of medications was 4 (range 0-39). Forty-nine percent of patients received ≥5 medications. In patients with polypharmacy, median number of medications was 8 (range 5-39) vs. 2 (range 0-4) among patients without polypharmacy. Patients with polypharmacy, compared to those without polypharmacy, were more likely to be ≥60 years (77% vs 42%, p<0.0001), had Karnofsky performance status (KPS) of ≤80 (53% vs.26%, p<0.0001) and adverse cytogenetics (41% vs 27%, p=0.01). Median hematopoietic cell transplant (HCT) comorbidity index (HCT-CI) was 2 (range 1-4) in the polypharmacy group vs 1 (range 1-4) in the group without polypharmacy (p<0.0001). Patients with polypharmacy were less likely to receive intensive chemotherapy (56% vs 81%, p<0.0001) and undergo HCT (17% vs 37%, p<0.0001).
Patients with polypharmacy had worse OS compared to those without polypharmacy (hazard ratio [HR] 2.1, (95% confidence interval [CI] 1.6-2.6], p=0.03) (Figure 1). One-year OS of patients with polypharmacy vs. those without polypharmacy was 29% vs 49% (p<0.001) (Table 1). OS was significantly lower with polypharmacy in patients <60 years of age (37% vs 65% at 1-year, p<0.001) but similar in patients ≥60 years (26% vs 27% at 1-year, p=0.09) (Figure 2 and 3). In polypharmacy group, 1-year OS for patients ≥60 years was 26% compared to 37% for patients <60 years (p=0.02). In two separate multivariate analyses, polypharmacy conferred worse OS in patients <60 years of age (HR 1.9, 95% CI 1.2-3.1) but not in patients ≥60 years (HR 1.1, 95% CI 0.8-1.6).
Conclusion
Patients with polypharmacy were older and had greater incidence of adverse cytogenetics, and were less likely to receive intensive chemotherapy and HCT. While HCT CI was slightly greater in the polypharmacy group, the difference in HCT CI does not seem to be clinically remarkable for the degree of difference in the median number of medicines between groups. AML patients with polypharmacy, when adjusted for age, had worse OS than those without polypharmacy. In multivariate analysis based on the age cutoff of 60 years, polypharmacy had negative impact on OS of patients aged <60 years but not on patients aged ≥60 years. While traditionally older adults are considered to be at a greater risk of polypharmacy, our study highlights that the effect of polypharmacy may be even more important in younger adults with AML. Given the impact of polypharmacy on OS, patients with newly diagnosed AML should be assessed for appropriateness of prescribed and over the counter medication.
Al-Kadhimi:Celldex Biotech: Other: Stocks; Seattle Genetics: Other: Stocks. Gundabolu:Pfizer: Consultancy; Novartis: Consultancy; Jazz pharmaceuticals: Consultancy; Samus Therapeutics: Research Funding. Bhatt:National Marrow Donor Program: Consultancy; Tolero Pharmaceuticals: Research Funding; Incyte: Consultancy, Research Funding; Partner therapeutics: Consultancy; Abbvie: Consultancy; Agios: Consultancy; CSL Behring: Consultancy; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract