Background: Enoxaparin given once daily (QD) for thrombotic disorders is less burdensome than twice-daily (BID) dosing. However, long-term outcomes when enoxaparin given as QD monotherapy are unknown.
Methods: We did a population-based cohort study. New users of enoxaparin alone (2005-2014) were identified in the linked healthcare databases of Quebec, Canada, and followed up for up to one year. The number of dispensed syringes divided by prescription length determined QD or BID enoxaparin by intention to treat. Cumulative rates of major bleeding and re-treatment with anticoagulants at one year were compared between enoxaparin groups. Re-treatment was initiation of anticoagulation after at least 30 days of no dispensed anticoagulants. The duration of enoxaparin monotherapy was the sum of prescriptions until discontinuation.
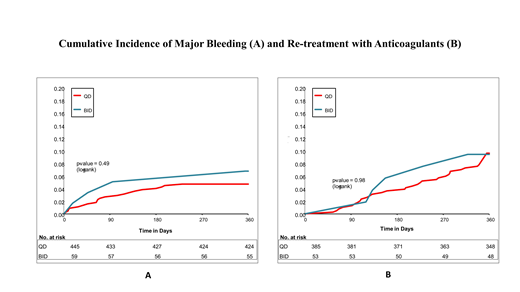
Results: The cohort included 504 patients; QD and BID enoxaparin users were 445 and 59, respectively. Mean (SD) age was 78.0 (6.6) years, 43.8% were males, and 61.9% had cancer. At 12 months, major bleeding occurred in 21 (4.7%) and 4 (6.8%) among QD and BID enoxaparin users, respectively (Figure 1A, P = 0.49). Re-treatment with anticoagulants occurred in 37 (9.6%) and 5 (9.4%) of QD and BID users, respectively (Figure 1B, P = 0.98). The duration of enoxaparin monotherapy was on average 13.9 (95% CI, 4.4-23.4, P = 0.005) days longer with QD vs BID use.
Conclusions: Monotherapy with QD enoxaparin was common and longer than BID enoxaparin with no apparent differences in bleeding or re-treatment with anticoagulants.
Klil-Drori:Sanofi Canada: Research Funding. Nazha:Sanofi Canada: Employment. Gharib:Sanofi Canada: Employment. Perreault:Sanofi Canada: Research Funding. Tagalakis:Servier: Other: participated on ad boards; Bayer: Other: participated on ad boards; Pfizer: Other: participated on ad boards; Sanofi Aventis: Other: investigator initiated grant;participated on ad boards; BMS-Pfizer: Other: participated on ad boards.
Author notes
Asterisk with author names denotes non-ASH members.