Background
Patients with Chronic Lymphocytic Leukemia (CLL) have an increased risk of infections both prior to and upon treatment. Infections are the major cause of death for these patients, the 5-year incidence of severe infection prior to treatment is approximately 32 % with a 30-day mortality of 10 % (Andersen et al., Haematologica, 2018).
Chemoimmunotherapy is still 1st line standard of treatment for patients without del17p or TP53 mutation despite association with neutropenia, immunesuppression and infections. The combination of BTK inhibitors and the bcl-2 inhibitor venetoclax has demonstrated synergy in vitro and in vivo, while translational data indicate that the CLL-related immune dysfunction can be improved on treatment with reduced risk of infections.
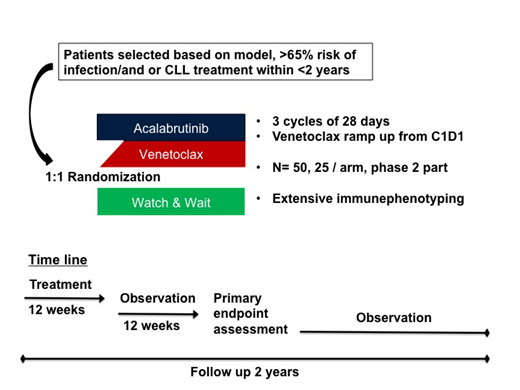
Employing the Machine-Learning based CLL treatment infection model (CLL-TIM) that we have developed, patients with a high (>65%) risk of infection and/or need of CLL treatment within 2 years of diagnosis can be identified (CLL-TIM.org). The significant morbidity and mortality due to infections in treatment-naïve CLL warrants trials that challenge the dogma of only treating symptomatic CLL. Thus, we initiated the randomized phase 2 PreVent-ACall trial of 12 weeks acalabrutinib + venetoclax to reduce risk of infections. Methods
Design and statistics
A phase 2, randomized, open label, multi-center clinical trial for newly diagnosed patients with CLL. Based on the CLL-TIM algorithm, patients with high risk of severe infection and/or treatment within 2 years from diagnosis can be identified. Approximately 20% of newly diagnosed CLL patients will fall into this high-risk group. First patient in trial planned for September 2019, primary outcome expected in 2021. Only patients identified as at high risk, who do not currently fulfil IWCLL treatment criteria are eligible. Patients will be randomized between observation in terms of watch&wait according to IWCLL guidelines or treatment.
Primary endpoint
Grade ≥3-Infection-free survival in the treatment arm compared to the observation arm after 24 weeks (12 weeks after end of treatment).
Study treatment
Acalabrutinib 100 mg BID from cycle 1 day 1 for 12 weeks.
Venetoclax, ramp up during the first five weeks starting cycle 1 day 1, thereafter 400 mg once daily for a total of 12 weeks counted from cycle 1 day 1.
Patients
A sample size of 25 patients in each arm, 50 patients in total.
Major inclusion criteria
CLL according to IWCLL criteria ≤1 year prior to randomization
High risk of infection and/or progressive treatment within 2 years according to CLL-TIM algorithm
IWCLL treatment indication not fulfilled
Adequate bone marrow function
Creatinine clearance above 30 mL/min.
ECOG performance status 0-2.
Major exclusion criteria
Prior CLL treatment
Richter's transformation
Previous autoimmune disease treated with immune suppression
Malignancies other than CLL requiring systemic therapies or considered to impact survival
Requirement of therapy with strong CYP3A4 and CYP3A5 inhibitors/inducers or anticoagulant therapy with vitamin K antagonists
History of bleeding disorders, current platelet inhibitors / anticoagulant therapy
History of stroke or intracranial hemorrhage within 6 months
Trial registry number
EUDRACT NUMBER: 2019-000270-29
Clinicaltrials.gov number: NCT03868722
Perspectives:
As infections is a major cause of morbidity and mortality for patients with CLL prior to any treatment, we aim at changing the natural history of immune dysfunction in CLL. The PreVent-ACaLL trial includes an optional extension into a phase 3 part with the primary outcome of grade ≥3 infection-free, CLL treatment-free survival two years after enrollment to address the unmet need of improved immune function in CLL for the first time.
Da Cunha-Bang:AstraZeneca: Consultancy; Janssen: Consultancy; Abbvie: Consultancy, Other: Travel Grant; Roche: Other: Travel Grant. Levin:Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Educational Grant; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Educational Grant; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Educational Grant; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Educational grant ; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Educational grant . Österborg:BeiGene: Research Funding; Gilead: Research Funding; Janssen: Research Funding; Abbvie: Research Funding; Kancera AB: Research Funding. Niemann:Novo Nordisk Foundation: Research Funding; Gilead: Other: Travel grant; Janssen: Consultancy, Other: Travel grant, Research Funding; Roche: Other: Travel grant; CSL Behring: Consultancy; Acerta: Consultancy, Research Funding; Sunesis: Consultancy; Astra Zeneca: Consultancy, Research Funding; Abbvie: Consultancy, Other: Travel grant, Research Funding.
acalabrutinib and venetoclax in combination for CLL.
Author notes
Asterisk with author names denotes non-ASH members.