Introduction:Diffuse large B cell lymphoma (DLBCL) is the most common type of Non-Hodgkin lymphoma (NHL). Treatment has improved over the years and can achieve 70% of survival (OS) in five years. Despite all progress in this field, central nervous system infiltration (CNSi), which is a devastating complication, still occurs with an incidence of 2-10%. Therefore, defining high-risk patients who consequently need prophylaxis seems to be the most adequate approach.
Objective: Evaluate central nervous system international prognostic index (CNS-IPI) score regarding reproducibility and validity of the model in a heterogeneous cohort outside of clinical trial.
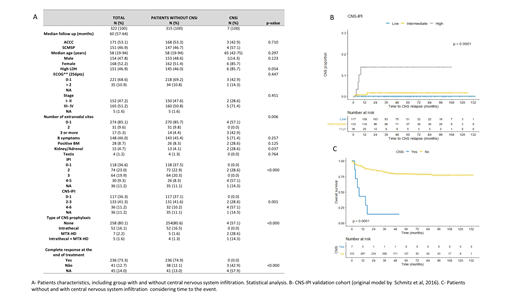
Patients and methods: This retrospective research study was approved by the Santa Casa de Misericórdia de São Paulo Medical School (SCMSP) and Ac Camargo Cancer Center (ACCC) Ethics Committee. Patients were eligible if they were sequentially diagnosed with primary DLBCL, between January 2007 and January 2016. Also, if patients had complete chart data, pathological review and had started treatment at the respective institution (SCMSP or ACCC). Lymphoma staging was defined according tothe Ann Arbor system and patients were also categorized by the international prognostic index - IPI at baseline All patients were treated with rituximab-based chemotherapy, mostly R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone).CNSi was diagnosed by liquor (positive cytology and/or immunophenotype), computerized tomography, magnetic resonance image and/or fluorodeoxy-glucose-positron emission tomography, requested only in symptomatic patients when CNSi was clinically suspected. Confirmed cases were classified as parenchymal, leptomeningeal or both. Validation of the CNS‐IPI was assessed by graphical comparison between our results of Kaplan‐Meier curves (risk stratification groups: low, intermediate and high risk) and those previously defined by CNS‐IPI.
Results:After inclusion and exclusion criteria 322 patients were available for the analysis (55% at ACCC vs. 46% at the SCMSP). Median follow-up was 60 months and median age was 58 years. There was similar distribution between male and female (47.8 vs. 52.2, respectively), and also similar distribution between localized (stage I-II) and advanced disease (stage III-IV), 47.2 vs. 51.2%, respectively. More than half of patients were low or low-intermediate IPI (59.6%) and low and intermediate CNS-IPI 0-3 (77.6%). The minority was submitted to CNS prophylaxis (19.9%), and the majority achieved complete response at the end of therapy (73.3%). Seven patients experienced CNSi, characterizing an incidence of 2.17% (7/322). The median time from diagnosis of DLBCL to CNSi was six months (range 2-52 months), while the median time of CNSi to death was 19 days (range 0 days -9.3 months). In our cohort, given the small number of events (7/322), an univariate model for risk factors was not possible, even though we statistically compared the groups of patients without and with CNSi. In univariate analysis at baseline LDH, number of extranodal sites, IPI, kidney/ adrenal were statically different in patients without and with CNSi (Table 1). Furthermore, regarding treatment, absence of complete response was also statistically different. CNS-IPI model stratified patients in a three-risk group model (low [0-1 points], intermediate [2-3 points] and high risk [4-6 points]) and demonstrateda 2-year rate of CNS relapse of 0.6%, 3.4% and 10.2%, respectively. In our cohort, using the same stratification, we obtained the2-year rate of CNS relapse of 0.0%, 0.8% and 13.8%, respectively. There was a statistical survival difference between the cohort of patients without or with CNSi. Median OS for patients without CNSi was not reached (average: 9.0 years [95% CI:8.5-9.5]) while median OS for patient with CNSi was of 8 months (95% CI: 2.8- 13.1). Log-rank p=0.000.
Conclusion:CNSi in patients with DLBCL treated with rituximab based chemotherapy remains a rare but devastating complication. Our study reinforces the validity and reproducibility of CNS-IPI, specific outside of clinical trials. Also guarantee the safety of CNS-IPI use in daily practice. Our results demonstrate that almost 80% of patients are low or intermediate risk of CNSi with no need of interventions. Those considered high risk would benefit from more intensive diagnosis and prophylaxis approach.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.