BACKGROUND. Patients (pts) with diffuse large B-cell lymphoma (DLBCL) refractory to second-line therapy or relapsed after an autologous stem cell transplant (ASCT) have a very poor clinical outcome with a median overall survival (OS) of 5 and 8-10 months, respectively. Autologous anti-CD19 chimeric antigen receptor (CD19 CAR) T cells have been associated with sustained complete remissions and long-term survivals in a large proportion of pts with R/R DLBCL by the two pivotal clinical trials Zuma1 and Juliet. This has led to the rapid approval by FDA and then by EMA of CAR-T cells for the third-line treatment of R/R DLBCL. Despite being a potentially revolutionary treatment for pts with advanced disease, the costs are much greater than any previously approved cancer therapy and this may become a substantial economic challenge for the health care system. The definition of inclusion and exclusion criteria capable of identifying more precisely pts who can successfully undergo CAR-T cell therapy, minimizing the severity of the toxicity, still remains a matter of discussion. Moreover, some eligible pts run the risk of becoming ineligible because of poor disease control. Indeed, one of the major obstacles to the successful use of CAR-T cells is the 4-5 week period so far required for the manufacturing and transfer of CAR-T cells. To address this issue, we have examined data of R/R DLBCL pts managed between 2010 and 2018 at our Center in order to: 1) better identify the characteristics and outcome of a cohort of R/R DLBCL pts potentially eligible, according to the approval criteria, for CAR-T cell therapy; 2) define factors influencing CAR-T cell eligibility; 3) make a realistic estimate of pts eligible for CAR-T cells.
METHODS. All DLBCL pts treated at our Center with R-CHOP were recorded and those who then subsequently underwent a second or subsequent line of therapy were included in our analysis. This cohort of R/R DLBCL was reviewed under IRB approval to determinate the potential eligibility to CAR-T cell therapy by applying the Juliet clinical trial inclusion/exclusion criteria. OS was defined as the time of interval from the second relapse until death from any cause or last follow-up. OS curves were estimated according to the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariable analyses were performed using the Cox proportional hazard model. Model selection was performed in a stepwise fashion. Conditional survival at the threshold of 28 days was predicted using the final multivariate Cox regression model after estimation of the baseline hazard through a Nelson-Aalen estimator. OS curves were estimated with the Kaplan-Meier method and compared using the log-rank test.
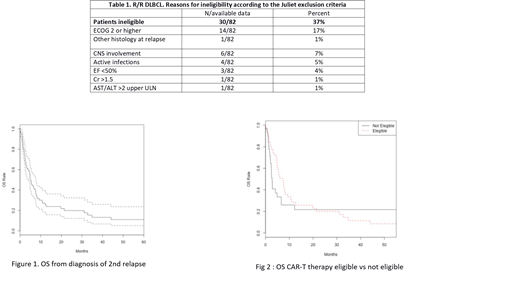
RESULTS. We have analyzed 116/480 (24%) pts with R/R DLBCL after R-CHOP managed between January 2010 and May 2018. Of these, 82/116 (71%) had received at least two lines of treatment and were further investigated. Median age was 64 years (21-87), 13 had relapsed after an ASCT, 7 within 1 year. Thirty of the 82 pts (37%) were defined as ineligible for CAR-T cell therapy by restrospective review, for reasons reported in Table 1. The median OS was 7 months in eligible vs 2 months in non-eligible pts (p=0.3). The 1-year OS was 27% in the overall pts population. In univariate analysis, OS was significantly reduced in pts with: B symptoms (p=.026), ECOG ≥2 (p=<.001), more than three lines of therapy (p=.048), elevated LDH (p=.001), comorbidities (p=.033). Multivariate analysis identified elevated LDH (p=0.019) and ECOG ≥2 (p=<.001) as significant prognostic factors for OS. Moreover, with regard to the feasibility of undergoing CAR-T cell therapy in this context, considering the required manufacturing time, we could estimate that pts without an elevated LDH and an ECOG ≥2 had a 28 day OS of 99%, compared to a 28 day OS of 88% for pts with both these factors.
CONCLUSIONS. In this retrospective real-life cohort of R/R DLBCLs, 82/480 pts (17%) were R/R tosecond-line treatment including ASCT. Considering Juliet's inclusion/exclusion criteria for CAR-T cell therapy, only 50 pts (10.4%) would be eligible for CAR-T cells. Our analysis suggests that elevated LDH plus ECOG ≥2 have to be considered the two most significant features of very rapid disease progression. These variables should be taken in account in order to better select DLBCL pts potentially eligible to CAR-T therapy.
Di Rocco:Roche: Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau; Sandoz: Consultancy. Martelli:Servier: Honoraria; F. Hoffman-La Roche, Celgene, Janssen, Sandoz, Novartis, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffman-La Roche, Celgene, Janssen, Sandoz, Novartis, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria. Foà:Roche: Consultancy, Speakers Bureau; Amgen Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.