Background: Nodal and splenic marginal zone lymphomas (NMZL/SMZL) are rare, usually disseminated, and typically diagnosed in older patients (median age 69 years). They are increasingly treated with first-line bendamustine/rituximab (BR) based on phase 3 trials that pooled various B-cell indolent lymphomas (Rummel et al., Lancet 2013). However, NMZL and SMZL have unique biology and clinical course. In this context, without histology-specific evidence, the risk/benefit ratio of BR over single-agent rituximab (R) in SMZL/NMZL is uncertain. Our objective was to examine treatment patterns, and to compare survival and toxicity outcomes of BR versus (vs.) R, using population-based data.
Methods: Using Medicare claims linked to cancer registry data covering ~34% of the US population (SEER-Medicare), we identified all SMZL/NMZL patients age ≥65 who received 1st line R or BR between 2009-2016 (after approval of bendamustine) and had complete Medicare records. We used a propensity score to generate "pseudo-randomized" cohorts (pooled, including NMZL and SMZL, and histology -specific for NMZL and SMZL), balancing patient and disease characteristics: age, sex, race, comorbidities (including kidney disease and heart failure), performance status, lymphoma stage, B symptoms, prior splenectomy, anemia (including autoimmune hemolytic), transfusions, hospitalizations, and time from diagnosis to therapy. In the balanced pseudo-randomized cohorts, we compared event-free survival (EFS, defined as 2nd line chemotherapy, splenectomy, hospice, or death) and overall survival (OS) from the start of therapy, risk of major toxicities (hospitalization, transfusions), and inflation-adjusted Medicare spending. Outcome models report hazard ratios (HR) or risk ratios (RR) with 95% confidence intervals (95%CI), and marginal means for costs.
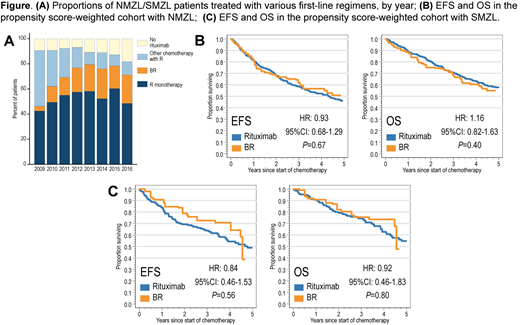
Results: Among 1,315 patients (901 with NMZL and 414 with SMZL), median age was 78 years [y], and there were 52% women. About half of patients received first-line R every year, but the proportion treated with BR increased from 4% in 2009 to 23% in 2016 (Fig. A).
In the pooled NMZL/SMZL cohort of patients receiving BR or R (N=926), those treated with BR were on average younger, more likely to have NMZL, stage 2-4 lymphoma, and B symptoms. With median follow-up 3.8 y, median EFS was 4.3 y (95%CI, 3.5-5.1) for NMZL, and 4.5 y (95%CI, 3.8-6.5) for SMZL. Median OS was 5.7 y (95%CI, 4.9-6.7) in NMZL, and 5.2 y (95%CI, 4.4-6.7) in SMZL. After balancing the confounders, there was no significant difference in either EFS (HR for BR, 1.00; 95%CI, 0.76-1.30, P=.98) or OS (HR, 1.14; 95%CI, 0.85-1.53; P=.38). Toxicities were higher with BR, including hospitalizations (RR, 1.46; 95%CI, 1.14-1.87; P=.003) and transfusions (RR, 2.35; 95%CI, 1.55-3.56; P<.001). Mean Medicare spending within 1 year from diagnosis was higher after BR (mean $83,480) than R (mean $53,776; P<.001). A sensitivity analysis showed that >50% of BR patients would need to have an unobserved risk factor doubling their mortality to explain lack of OS benefit.
We then fitted separate propensity score models in NMZL and SMZL. In the comparative NMZL cohort (N=609), 434 patients (71%) received R, and 175 (29%) received BR. We observed no significant difference in either EFS (HR for BR, 0.93; 95%CI, 0.68-1.29; P=.67; Fig. B) or OS (HR, 1.16; 95%CI, 0.82-1.63; P=.40), whereas toxicities and costs were consistently higher with BR. In the comparative SMZL cohort (N=317), 272 patients (86%) received R, and 45 (14%) received BR. Again, there was no significant difference in EFS (HR for BR, 0.84; 95%CI, 0.46-1.53; P=.56; Fig. C) or OS (HR, 0.92; 95%CI, 0.46-1.83; P=.80). Results for toxicities and costs were again consistent.
Conclusions: Among older patients with SMZL and NMZL treated in the community, the use of first-line BR is increasing. However, in this large observational study, BR was not associated with any significant EFS or OS benefit over single-agent R, while it increased toxicities and costs. Without a formal randomized histology-specific study, single-agent R may offer a favorable risk/benefit ratio as a first-line therapy for older NMZL/SMZL patients at average risk, reserving BR for those with compelling clinical circumstances. Our results may however be affected by bias due to unobserved confounding, and may not be applicable to younger patients.
Olszewski:Adaptive Biotechnologies: Research Funding; Genentech: Research Funding; TG Therapeutics: Research Funding; Spectrum Pharmaceuticals: Research Funding.
Rituximab with bendamustine is off-label for marginal zone lymphoma
Author notes
Asterisk with author names denotes non-ASH members.