Background: The paradigm for treatment selection in newly diagnosed (ND) multiple myeloma (MM) is largely predicated on establishment of suitability for front-line autologous stem cell transplantation. In this context, real-world evidence from multiple jurisdictions demonstrates that the most widely employed front-line (1L) therapies are bortezomib (B)-based triplets in transplant-eligible patients, and in transplant-ineligible patients, B-based triplets (or doublets) or the combination of lenalidomide and dexamethasone. While a sound hypothetical rationale for treatment stratification at diagnosis is recognised, viz alternative treatments for high-risk (HR) versus standard-risk disease, this is rarely employed in either everyday practice or clinical trials. A significant impediment to such an approach is the lack of a uniformly recognised risk stratification model that not only recognises truly HR disease (e.g. median survival from diagnosis < 24 months [m]) but is also accessible and affordable. Against this background we evaluated data from the Australian and New Zealand Myeloma and Related Diseases Registry (MRDR) to define characteristics of 'functional' HR disease that could inform response-adaptive strategies, thus enabling the appropriate therapeutic targeting of HR patients.
Methods: NDMM patients enrolled on to the MRDR since February 2013 with available data were evaluated. Patients were defined as either sub-optimal responders (SOR) to 1L if their best response was minimal response or stable disease (but not progressive disease [PD]), or early progressors (EP) if they demonstrated PD within 12m of commencing 1L therapy, irrespective of whether this occurred on or after the completion of 1L therapy. For categorical variables p-values were determined using a Pearson's chi-squared test, for continuous variables p-values were calculated using a Wilcoxon rank-sum test. Survival analysis was performed using the Kaplan-Meier method and p-values determined with the log-rank test.
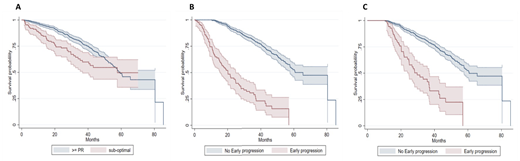
Results: 1320 NDMM patients were included with a median follow-up of 23m (12-37 IQR, 0-83 range). Of these, 152 were SOR (11.5%) and 118 were EP (8.9%). At diagnosis only 2 factors associated with SOR were identified: age >70y (p<0.001) and 1L therapy not containing B (p<0.001), with logistic regression confirming that these were independently associated with SOR: age >70y OR 1.59 (95% CI 1.11-2.28; p=0.01) and B-based 1L OR 0.47 (95% CI 0.29-0.75; p=0.001). Median overall (OS) survival for SOR was similar to that of responders (R): 57.8m (95%CI, 36.7 - NR) vs 59.3m (95%CI, 55.4 - NR), for SOR vs R, respectively, but with an excess of early deaths from the time of diagnosis in a sub-set of SOR patients: survival at 12m 85% vs 96%, 24m 73% vs 88% and 36m 61% vs 79%, for SOR vs R, respectively, (HR 1.6, 95% CI 1.2 - 2.3; p=0.001) (Figure A). Conversely, EP was associated with a range of indicators of higher-disease burden: higher ISS (p<0.001), higher R-ISS (p<0.001), inferior ECOG (p = 0.007) and the presence of CRAB criteria - hypercalcaemia (p=0.002), renal insufficiency (p<0.001) and anaemia (p<0.001). Importantly, 25 % of EP demonstrated a SOR compared to only 11% of late progressors, p<0.001. Refractory disease at time of first relapse (1R) was also significantly higher in EP vs non-EP: 41.8% vs 17.0%, p<0.001, respectively. OS, whether measured from diagnosis (Figure B) or utilising a 12m landmark analysis (Figure C) demonstrated markedly inferior OS for EP vs non-EP: median OS 20.2m (95%CI, 15.3 - 25.0) vs 60.7m (95%CI, 57.7 - NR, p<0.001; HR 6.7, 95% CI 5.1 - 8.7) and 29.7m (95%CI, 24.7 - 36.8) vs 60.7m (95%CI, 57.7 - NR; p<0.001; HR 4.0, 95% CI 2.8 - 5.6), respectively.
Conclusions: These real-world data demonstrate that 40% of patients with SOR will die within 3y of diagnosis. More strikingly, EP is associated with high disease burden at diagnosis and high likelihood of refractoriness at 1R, with median OS of only 20.2m. While recent data demonstrate that 1L triplets or quadruplets containing the monoclonal antibody daratumumab may mitigate against both SOR and EP, these approaches remain financially implausible in most jurisdictions. A more affordable approach could be informed by response-adaptive trials in functional HR MM in an attempt to address the ongoing unmet therapeutic need in these patient groups.
Spencer:Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Specialised Therapeutics Australia: Consultancy, Honoraria; Secura Bio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Haemalogix: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Mollee:Janssen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Blacklock:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Harrison:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: investigator on studies, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Research Funding; Janssen Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Quach:Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Ho:Celgene: Other: investigator meeting travel costs; Janssen: Other: investigator meeting travel costs; Novartis: Other: investigator meeting travel costs; La Jolla: Other: investigator meeting travel costs. Spearing:Celgene: Consultancy, Honoraria, Other: Travel costs; Roche: Other: Travel costs. Prince:Amgen: Consultancy, Honoraria; Celgene: Honoraria; Takeda: Consultancy, Honoraria; Allergan: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Moore:Gilead: Research Funding; Takeda: Research Funding. McQuilten:Takeda Pharmaceuticals: Research Funding; AbbVie: Research Funding; Janssen-Cilag: Research Funding; Celgene: Research Funding; Gilead Sciences: Research Funding; CSL Biotherapies: Research Funding. Wood:Roche: Research Funding; Gilead: Research Funding; Takeda: Research Funding; Amgen: Research Funding; Abbvie: Research Funding; Janssen-Cilag: Research Funding; Novartis: Research Funding; Sanofi: Research Funding; Bristol-Myers Squibb: Research Funding; Alexion: Research Funding; Celgene: Research Funding; CSL Behring: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.