Background:
Despite recent advances, outcome of patients with AML, particularly the older ones, remains poor. This is in part because of adverse features more frequently associated with AML in this patient population. Older patients with AML have high mortality (>90%). This is driven by the fact that over 50% of patients will experience a relapse, and most relapsed patients will die from AML within a year. There is no consensus standard treatment for relapsed or refractory disease, highlighting the high unmet need for these patients. Devimistat is a novel lipoic acid analogue that inhibits pyruvate dehydrogenase (PDH) and α-ketogluterate dehydrogenase. This inhibits mitochondrial respiration and cause hyper-phosphorylation of PDH and activation of adenosine monophosphate activated kinase (AMPK) in AML cells. The ARMADA 2000 trial seeks to leverage the unique mechanism of action of this agent to improve the outcomes for older patients suffering from relapsed or refractory AML. To date devimistat has been given to more than 108 relapsed or refractory AML patients in multiple clinical trials (phase I and phase II). These studies suggest that devimistat can be safely combined with high dose cytarabine and mitoxantrone in relapsed or refractory AML patients. The possible beneficial effect in older patients was demonstrated by the dose response relationship seen in older but not younger patients. The combined efficacy result from 23 treated patients (≥ 60 years) on either of phase I or phase II studies of devimistat and high dose cytarabine and mitoxantrone (CHAM) showed complete remission (CR) rate of 48%, CR + CRi of 52% and median overall survival (OS) of 12.4 months [interim result of this study was presented at EHA Annual Meeting 2018, for further details please refer: Analysis of phase I and pilot phase II data reveal 2,000 mg/m2 as the optimal dose of CPI-613 in combination with cytarabine and mitoxantrone for elderly patients with relapsed or refractory AML]. Given the favorable safety profile of CHAM with the promising response results achieved in these trials, further evaluation of devimistat in AML is warranted.
The current study evaluates devimistat in combination with high dose cytarabine and mitoxantrone (CHAM) in older patients with relapsed or refractory AML.
Method:
This is a multicentre, open label, randomized phase III study of devimistat in combination with high dose cytarabine and mitoxantrone (CHAM) compared to high dose cytarabine and mitoxantrone (HAM) in older patients with relapsed/refractory AML.
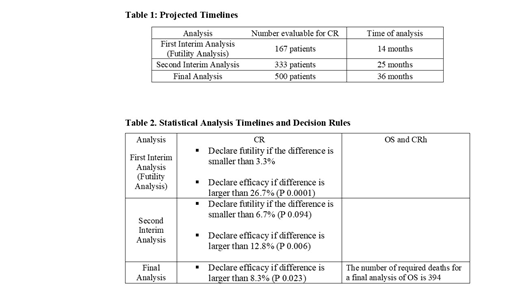
Eligible patients are male and female individuals who are 60 years and older with histologically documented AML that is relapsed from, or refractory to, prior standard therapies that include standard dose cytarabine or high dose cytarabine based induction cycle or no response after at least 3 cycles of a hypomethylating agent with or without venetoclax. Other key inclusion criteria include ECOG performance status 0-2 and expected survival >3 months. A total of 500 patients will be randomized in a 1:1 fashion between arms. Following completion of all planned induction and/ or consolidation therapy cycles, patients in remission on the CHAM arm will continue to receive devimistat during maintenance cycle(s) until disease recurrence, availability of stem cell transplant, the advent of intolerable side effects, or patient withdrawal of consent. Primary endpoint of the study is complete remission (CR) of CHAM compared to HAM. Secondary endpoints include overall survival (OS), complete remission with partial hematologic recovery (CRh) and safety. Exploratory analysis will examine the expression of a gene signature from baseline marrow samples found to be predictive of response in the phase I study. Additional analysis will correlate the expression of several key proteins including PDH, KGDH, PDK1-4, SOD2 and CD79a in baseline marrow samples with response. Statistical analysis plan for this trial is summarized in Table 1 and Table 2. This study was initiated in November 2018 and planned at approximately 87 sites in more than 13 countries, recruiting 500 patients. The interim analysis of the study is expected to be completed as early as Q3 2020. Clinical trial information: NCT03504410
Pardee:Rafael Pharmaceuticals: Consultancy, Research Funding; Karyopharm: Research Funding; Pharmacyclics/Janssen: Speakers Bureau; Celgene: Speakers Bureau; Amgen: Speakers Bureau; CBM Bipharma: Membership on an entity's Board of Directors or advisory committees; Spherix Intellectual Property: Research Funding. Luther:Rafael Pharmaceuticals: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Buyse:Rafael Pharmaceuticals: Consultancy. Powell:Rafael Pharmaceuticals: Consultancy, Research Funding; Novartis: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding; Janssen: Research Funding. Cortes:Bristol-Myers Squibb: Consultancy, Research Funding; BiolineRx: Consultancy; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Sun Pharma: Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding.
Devimistat is not approved by the FDA for any indication and the clinical trial describes its use in AML.
Author notes
Asterisk with author names denotes non-ASH members.