Introduction
Both venous thromboembolism (VTE) and intracranial hemorrhage (ICH) are common potentially life-threatening complications of primary and metastatic brain tumors. Despite emerging evidence regarding the safety of anticoagulation in patients with brain tumors, there is little evidence on appropriate management of VTE following an ICH. Potential management options after an ICH in patients with brain tumors include resumption of full or modified dose anticoagulation or cessation of anticoagulant therapy with or without placement of an inferior vena cava (IVC) filter. We evaluated rates of recurrent VTE and ICH following an initial ICH occurring on anticoagulant therapy.
Methods
A retrospective cohort study was performed using a hospital-based online medical record database (CQ2) which links ICD-9 and ICD-10 codes with prescription medication records. Cases were identified based on coding for primary brain tumors or brain metastases, after which charts were manually reviewed for a diagnosis of ICH. A blinded review of radiographic imaging was performed, and the initial ICH was categorized as either trace, measurable, or major. Measurable intracranial hemorrhages were those defined as greater than 1 mL in volume and major intracranial hemorrhages were defined as greater than 10 mL in volume, symptomatic, or requiring surgical intervention. The electronic medical record was reviewed to ascertain longitudinal anticoagulation status after the initial ICH. The primary endpoints of the study were recurrent ICH and venous thromboembolism (VTE) within 12 months from the initial ICH. Gray's test was used to compare the cumulative incidence of recurrent ICH and VTE between the groups, with death as a competing risk.
Results
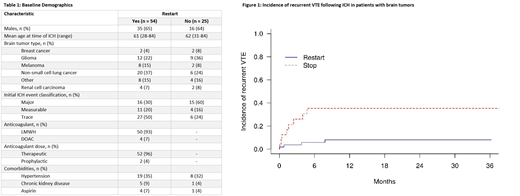
A total of 79 patients with primary brain tumors or brain metastases and confirmed ICH were included in the study. Fifty-four patients (68.4%) restarted anticoagulation after ICH and 25 patients discontinued anticoagulation entirely. The cohorts were well-matched for tumor diagnosis, age, and comorbidities that portend an increased risk of ICH such as hypertension, chronic kidney disease, and concomitant aspirin use (Table 1).
The cumulative incidence of recurrent ICH (95% CI) at one year was 6.1% (1.5 - 15.3) in the restart cohort compared to 4.2% (0.3 - 18.3) in patients who did not restart anticoagulation. Median time from anticoagulation restart to recurrent ICH was 36 days. A total of 16 of 31 patients with major ICH restarted anticoagulation and among these patients two developed subsequent ICH (cumulative incidence 14.5%, 95% CI 2.1 - 38.3). Among the 15 patients with a major ICH who did not restart anticoagulation, the cumulative incidence was 6.7% (0.3 - 27.5). Eleven of 15 patients with measurable ICH restarted anticoagulation and among these patients one subsequently developed ICH (cumulative incidence 0.1%, 95% CI 0.0 - 0.3). No recurrent ICH events were observed in 33 patients with trace initial hemorrhages regardless of restart status. All recurrent ICH events met criteria for classification as a major hemorrhage on the basis of clinical symptoms, and 30-day mortality after recurrent ICH was 100%.
The cumulative incidence of recurrent VTE was significantly lower in the restart cohort compared to cohort of patients who did not restart anticoagulation (8.1 vs. 35.3, P=0.003, Figure 1). There were a total of five VTE events in the restart cohort, three deep vein thrombi (DVT) and two pulmonary emboli (PE). Two of the DVT were associated with an IVC filter. There were a total of nine VTE events in patients who did not restart anticoagulation, seven DVT and two PE. Five of the DVT were associated with an IVC filter. The two PE were both submassive events requiring ICU admission.
Conclusions
Recurrent VTE events are less frequent and less severe in patients who restart anticoagulation following ICH in patients with brain tumors on anticoagulation. Restarting anticoagulation after smaller ICH (trace or measurable) appears safe. However, approximately 1 in 7 patients with major initial ICH who restarted anticoagulation subsequently developed recurrent major ICH that was associated with a very high mortality rate. This raises serious questions as to the safety of restarting therapeutic anticoagulation following major hemorrhage in the setting of brain tumors.
Neuberg:Pharmacyclics: Research Funding; Madrigal Pharmaceuticals: Equity Ownership; Celgene: Research Funding. Zwicker:Quercegen: Research Funding; Daiichi: Consultancy; Seattle Genetics: Consultancy; Parexel: Consultancy; Incyte: Research Funding; Bayer: Consultancy; Portola: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.