Introduction: Myelofibrosis (MF) is characterized by bone marrow fibrosis, splenomegaly, cytopenias, and poor survival. Ruxolitinib (RUX) is currently the only approved treatment (Tx) for intermediate- or high-risk MF. Clinical outcomes after RUX exposure are poor and no other Tx is available. Fedratinib (FEDR) is an oral, selective inhibitor of JAK2 investigated in the single-arm, phase II JAKARTA2 study in patients (pts) with intermediate- or high-risk MF previously exposed to RUX. The effect of FEDR on pt-reported MF-associated symptoms and HRQoL in JAKARTA2 was assessed.
Methods: All pts received FEDR at a starting dose of 400 mg/day in 28-day Tx cycles. Pt-reported symptoms were assessed daily during the week before day 1 of each Tx cycle and at the end of cycle 6 (EOC6) using the modified Myelofibrosis Symptom Assessment Form (MFSAF). The MFSAF comprises 6 key MF symptoms (night sweats, pruritus, early satiety, pain under ribs on the left side, abdominal discomfort, bone or muscle pain), each scored from 0 (absent) to 10 (worst imaginable). Total symptom score (TSS), ranging from 0-60, was estimated by averaging daily TSS (sum of all individual symptom scores) during the week of each scheduled assessment.
The EORTC Quality of Life Core 30 Questionnaire (QLQ-C30) measures QoL in pts with cancer. The QLQ-C30 has 15 HRQoL domains, including global health status/QoL and multiple functional and symptom domains. Domains are scored from 0-100; higher scores indicate better QoL or functioning, but worse symptomology. The QLQ-C30 was administered on day 1 of each cycle and at EOC6.
The Patient's Global Impression of Change (PGIC) evaluates pt perceptions of MF symptom severity over time, providing an overall sense of whether Tx is beneficial. Answers range from 1 (very much improved) to 7 (very much worse). The PGIC was administered on day 1 of cycles 4 and 6 and at EOC6.
The MFSAF and QLQ-C30 populations comprised all treated pts with an evaluable pt-reported outcome assessment measure at BL (defined as TSS data for ≥5 of 7 days on the MFSAF in the week before C1D1; and ≥15 of 30 items on the QLQ-C30 answered at BL). The PGIC population included all treated pts. Change from BL at each post-BL visit and corresponding effect size (95% confidence intervals [CI]) were estimated using Hedges' g. An effect size of 0.5 (medium; ie, half of the standard deviation) is a commonly used threshold for the clinical meaningfulness of a change from BL at a given visit when a threshold is not well established. Symptom response rate (≥50% reduction in TSS from BL) was estimated at each post-BL visit among pts with TSS >0 at BL and compared among clinically relevant pt subgroups. Pts with missing post-BL values were deemed nonresponders. Proportions of pts with clinically meaningful improvements in domains of the QLQ-C30 (≥10-point change from BL) were also evaluated across visits and compared between subgroups.
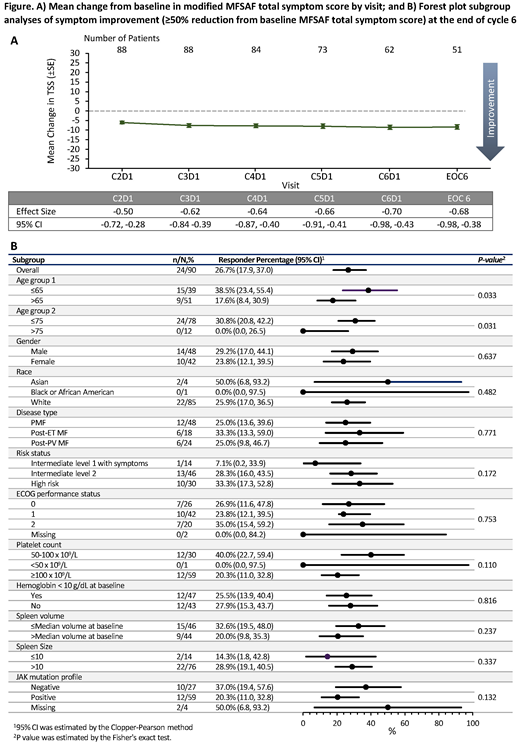
Results: The MFSAF evaluable population included 90/97 (93%) JAKARTA2 pts. Mean TSS at BL was 21. Clinically meaningful improvements from BL were observed across visits in TSS (Fig. A) and in most individual symptoms scores: effect sizes at EOC6 were -0.68 for TSS, -0.69 for early satiety, -0.68 for night sweats, -0.59 for pain under ribs on left side, -0.47 for abdominal discomfort, -0.43 for pruritis, and -0.28 for bone or muscle pain. Overall, 26.7% of pts had a symptom response. TSS improvements were observed across pt subgroups (Fig. B).
Pts had worse mean scores at BL across most QLQ-C30 domains than age- and gender-matched general populations. Most QLQ-C30 domains showed clinically meaningful (≥10-point change) improvement from BL across all or most post-BL visits in global health status/QoL, physical functioning, role functioning, fatigue, pain, dyspnea, insomnia, and appetite loss. About 44% (95%CI 30, 59), 53% (38, 68), and 39% (25, 54) of pts had clinically meaningful improvements at EOC6 in the global health status/QoL, physical functioning, and role functioning domains, respectively. Minimal heterogeneity of Tx effect was seen in subgroup analyses of global QoL. Most ongoing pts (>80%) reported overall symptom improvement on the PGIC.
Conclusion: Tx with FEDR in pts previously exposed to RUX resulted in clinically meaningful improvement from BL in global QoL and functional status, in addition to MF-associated symptoms. Global QoL and MF-associated symptom improvement was shown across clinically relevant pt subgroups.
Harrison:Promedior: Honoraria; Roche: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau; AOP: Honoraria; Celgene: Honoraria, Speakers Bureau; Sierra Oncology: Honoraria; Shire: Speakers Bureau; Janssen: Speakers Bureau; CTI: Speakers Bureau; Gilead: Speakers Bureau; Incyte: Speakers Bureau. Schaap:Celgene: Consultancy; Novartis: Consultancy. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ITALFARMACO: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Kiladjian:Celgene: Consultancy; Novartis: Honoraria, Research Funding; AOP Orphan: Honoraria, Research Funding. Jourdan:Novartis: Honoraria; Astellas: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Silver:PharmEssentia: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Schouten:Gilead: Honoraria; Alexion: Honoraria, Other: Travel expenses; Sanofi: Honoraria; Novartis: Honoraria. Passamonti:Celgene Corporation: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Zweegman:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Talpaz:NS Pharma: Research Funding; Celgene: Consultancy, Other: Travel; Stemline: Research Funding; Promedior: Research Funding; Samus: Research Funding; BMS: Consultancy; Gilead: Research Funding; Asana: Research Funding; Incyte: Research Funding; CTI: Research Funding; Janssen: Research Funding; Constellation: Research Funding; Novartis: Research Funding. Verstovsek:Incyte: Research Funding; Roche: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Gilead: Research Funding; Promedior: Research Funding; CTI BioPharma Corp: Research Funding; Genetech: Research Funding; Blueprint Medicines Corp: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Research Funding; Pharma Essentia: Research Funding; Astrazeneca: Research Funding; Ital Pharma: Research Funding; Protaganist Therapeutics: Research Funding; Constellation: Consultancy; Pragmatist: Consultancy. Rose:Celgene Corporation: Employment, Equity Ownership. Tang:Celgene Corporation: Employment, Equity Ownership. Hu:Celgene: Employment, Equity Ownership. Guo:Evidera: Employment. Liao:Evidera: Employment. Brownstein:Celgene: Employment, Equity Ownership. Mesa:PharmaEssentia: Research Funding; CTI: Research Funding; Pfizer: Research Funding; LaJolla: Consultancy; Samus: Research Funding; Genotech: Research Funding; Galena Biopharma: Consultancy; Promedior: Research Funding; Shire: Honoraria; Genentech: Consultancy; NS Pharma: Research Funding; Incyte: Other: travel, accommodations, expenses, Research Funding; AOP Orphan Pharmaceuticals: Honoraria, Other: travel, accommodations, expenses; Baxalta: Consultancy; Gilead Sciences: Research Funding; Sierra Oncology: Consultancy; Novartis: Consultancy, Honoraria, Other: travel, accommodations, expenses; AbbVie: Research Funding; Celgene Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.