Introduction: Glasdegib, an oral inhibitor of the Hedgehog (Hh) signaling pathway, is approved in the USA in combination with low-dose cytarabine (LDAC) to treat patients (pts) with newly diagnosed acute myeloid leukemia (AML) unable to receive intensive chemotherapy due to comorbidities or age (≥75 years). In a randomized trial, this combination significantly improved overall survival (OS) vs LDAC alone (Cortes et al. 2019). Here we report the efficacy and safety of glasdegib combined with azacitidine (AZA) in pts with higher-risk myelodysplastic syndromes (MDS), AML, and chronic myelomonocytic leukemia (CMML) similarly ineligible for intensive chemotherapy.
Methods: This open-label, multicenter, phase 1b trial (BRIGHT MDS & AML 1012; NCT02367456) enrolled pts with newly diagnosed higher-risk MDS, AML, and CMML (Start date, April 2015; estimated study completion date, January 2021). Pts received oral glasdegib (100 mg once daily) continuously in combination with AZA (75 mg/m2/day) on Days 1-7 of a 28-day cycle. Treatment was continued until disease progression, unacceptable toxicity, death, or pt refusal. The primary endpoint was complete remission (CR) using European LeukemiaNet (ELN) for AML/MDS International Working Group criteria per investigator assessment. Efficacy outcomes are reported as of June 17, 2019; all other outcomes are reported as of April 19, 2019.
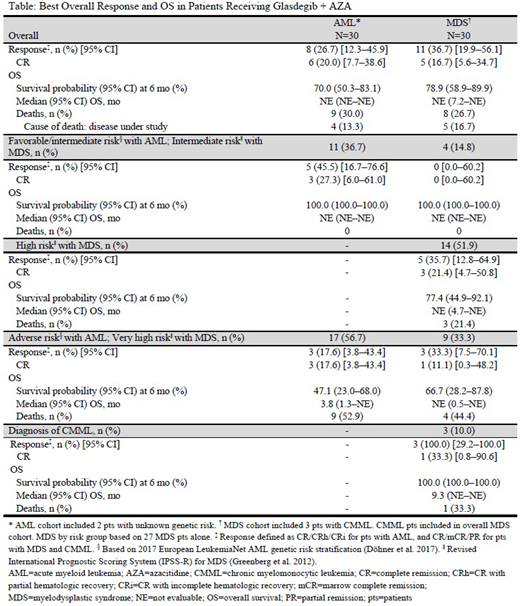
Results: Sixty pts (AML, n=30; MDS, n=30) were enrolled and received treatment with glasdegib + AZA. Among pts with AML, the median age was 74.0 years (range, 56-87), 40% were female; 7%, 30% and 57% of pts had a favorable, intermediate and adverse ELN genetic risk category, respectively. Median treatment duration was 19.2 weeks (range, 1.1-47.4); median follow-up was 7.8 (95% CI, 5.9-9.6) months. At the time of data cut-off, 43% of pts were still receiving glasdegib and/or AZA. The most frequent (>30% of pts) all-causality treatment-emergent adverse-events (TEAEs) of all grades were nausea (60%), decreased appetite (53%), constipation (50%), vomiting (43%), diarrhea (43%), and pyrexia (33%). The most frequent (>10% of pts) serious TEAEs were febrile neutropenia (20%) and pyrexia (13%). The 30-day mortality rate was 10%. Two of 23 pts (8.7%) had Cycle 2 delay due to adverse events (AEs). Eight (27%) pts achieved CR/CR with partial hematologic recovery/CR with incomplete hematologic recovery; 6 (20%) achieved CR. Median time to CR was 5.5 months (range, 3.1-6.0). Median OS was not evaluable (NE [95% CI, NE-NE]) and the 6-month survival probability was 70.0% (95% CI, 50.3-83.1%). Response and survival by risk category are shown in the Table.
Of the 30 pts with MDS (including 3 with CMML), the median age was 72 years (range, 55-89) and 20% were female; 15, 52, and 33% of pts had an intermediate, high, and very high Revised International Prognostic Scoring System risk, respectively. Median treatment duration was 19.6 weeks (range, 1.6-50.6); median follow-up was 6.9 months (95% CI, 6.5-10.9). At the time of data cut-off, 40% of pts were still receiving glasdegib and/or AZA. The most frequent (>30% of pts) all-causality TEAEs were nausea (67%), constipation (47%), diarrhea (40%), muscle spasms (40%), neutrophil count decreased (40%), anemia (37%), dysgeusia (37%), platelet count decreased (33%) and vomiting (33%). The most frequently (>10% of pts) reported serious TEAEs were febrile neutropenia (17%) and sepsis (17%). The 30-day mortality rate was 3%. Three of 26 pts (11.5%) had Cycle 2 delay due to AEs. Eleven (37%) pts achieved CR/marrow CR/partial remission; 5 (17%) achieved CR. Median time to CR was 5.6 months (range, 3.7-6.4). Median (95% CI) OS was NE months (7.2-NE) and the 6-month survival probability was 78.9% (95% CI, 58.9-89.9%). Response and survival by diagnosis and by risk category are shown in the Table.
Conclusions: The addition of glasdegib to AZA for pts with newly diagnosed higher-risk MDS, AML, or CMML ineligible for intensive chemotherapy showed promising rates of CR and OS. Glasdegib + AZA was generally well tolerated, with a safety profile consistent with toxicities of AZA monotherapy and other marketed inhibitors of the Hh signaling pathway. Glasdegib is currently in phase 3 clinical development for AML therapy in combination with AZA (NCT03416179). Further studies of glasdegib + AZA in pts with MDS are warranted.
Sekeres:Celgene: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees. Schuster:Amgen: Speakers Bureau; Janssen: Speakers Bureau; Novartis: Speakers Bureau; Incyte: Research Funding; Karyopharm Therapeutics: Research Funding; Morphosys: Research Funding; Nordic Nanovector: Research Funding; Pharmacyclics: Research Funding, Speakers Bureau; Rafael: Research Funding; F2G Ltd.: Research Funding; AbbVie: Speakers Bureau; Seattle Genetics: Speakers Bureau; Takeda: Speakers Bureau; Verastem: Speakers Bureau; Astellas: Speakers Bureau; Celgene: Speakers Bureau; Genentech: Speakers Bureau; Actinium: Research Funding. Krauter:Pfizer: Honoraria. Maertens:Astellas Pharma: Other: Personal fees and non-financial support; Pfizer: Other: Grant and personal fees; F2G: Other: Personal fees and non-financial support; Gilead Sciences: Other: Grants, personal fees and non-financial support; Merck: Other: Personal fees and non-financial support; Cidara: Other: Personal fees and non-financial support; Amplyx: Other: Personal fees and non-financial support. Gyan:Pfizer: Honoraria. Kovacsovics:Abbvie: Research Funding; Pfizer: Research Funding; Amgen: Consultancy, Research Funding; Novartis: Research Funding; Jazz: Consultancy. Verma:BMS: Research Funding; Janssen: Research Funding; Stelexis: Equity Ownership, Honoraria; Acceleron: Honoraria; Celgene: Honoraria. Vyas:Abbvie: Speakers Bureau; Daiichi Sankyo: Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Forty Seven, Inc.: Research Funding; Pfizer: Speakers Bureau; Celgene: Research Funding, Speakers Bureau; Astellas: Speakers Bureau. Wang:Abbvie: Other: Advisory role; Kite: Other: Advisory role; Jazz: Other: Advisory role; Astellas: Other: Advisory role, Speakers Bureau; celyad: Other: Advisory role; Pfizer: Other: Advisory role, Speakers Bureau; Stemline: Other: Advisory role, Speakers Bureau; Daiichi: Other: Advisory role; Amgen: Other: Advisory role; Agios: Other: Advisory role. Ma:Pfizer: Employment, Equity Ownership. Zeremski:Pfizer: Employment, Equity Ownership. Kudla:Pfizer: Employment, Equity Ownership. Chan:Pfizer Inc: Employment, Equity Ownership. Zeidan:Agios: Honoraria; Ariad: Honoraria; Cardinal Health: Honoraria; Novartis: Honoraria; Trovagene: Consultancy, Honoraria, Research Funding; Otsuka: Consultancy, Honoraria, Research Funding; Seattle Genetics: Honoraria; BeyondSpring: Honoraria; Incyte: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Medimmune/AstraZeneca: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Astellas: Honoraria; ADC Therapeutics: Research Funding; Celgene Corporation: Consultancy, Honoraria, Research Funding; Acceleron Pharma: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; Daiichi Sankyo: Honoraria.
Glasdegib is approved in the US in combination with low-dose cytarabine for treatment of newly diagnosed AML in patients not suitable for intensive chemotherapy due to comorbidities or age (75 years or older). Here we report data from a phase 1b trial where glasdegib was combined with azacitidine in patients with higher-risk myelodysplastic syndromes, AML, and chronic myelomonocytic leukemia similarly ineligible for intensive chemotherapy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract