Introduction: In the revised 2017 World Health Organization (WHO) classification of lymphoid neoplasms, high-grade B-cell lymphoma with C-MYC and BCL2 or BCL6 rearrangements is classified as Double-Hit lymphoma (DHL), while the term Triple Hit lymphoma (THL) is employed when the three genes are rearranged (Swerdlow SH, et al 2017). Amplification of C-MYC plus BCL2 and/or BCL6 rearrangement or amplification are defined as "atypical DHL and THL" (Li S., et al 2015). We will here collectively refer to DHL and THL as DHL. Both true and atypical DHL are resistant to conventional chemo-immunotherapy and are associated with poorer outcome. The median survival is shorter than 12 months with standard regimens (Snuderl M, et al 2010). The success of intensified chemo-immunotherapy protocols in Burkitt lymphoma, encouraged many Centres to include these regimens in the treatment armamentarium of DHL. Among these protocols, the B-ALL/NHL 2002 of the GMALL multicenter group is an effective and well tolerated regimen in the treatment of Burkitt's lymphoma (Pollen M, et al 2011). We have adopted the GMALL protocol, as our guideline for the treatment of patients with true or atypical DHL. The preliminary experience with the GMALL protocol in 18 patients is here reported.
Methods: We retrospectively evaluated patients with true or atypical DHL treated with the GMALL protocol at the European Institute of Oncology in Milan from 2015 and at the Mauriziano Hospital in Turin from 2012. The diagnosis was confirmed according to 2017 WHO classification and immunohistochemistry analysis and fluorescent in situ hybridization were performed in all patients. Patients were treated following the R-GMALL protocol according to their age group with less intensified doses applied to patients older than 55 years. The program was part of the treatment guidelines developed at each Center for the management of lymphoma patients and a written informed consent was obtained from all patients.
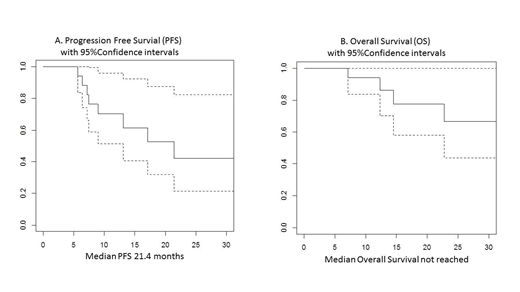
Results: Eighteen patients were included in this analysis, 14 were true DH and 3 atypical DH and 1 was Burkitt like lymphoma. Five were transformed lymphomas, 4 after follicular and 1 after marginal zone lymphoma. The median age of the whole group was 64 years (range 43-77years). Eleven patients were male (61%), 15 patients (83%) had Ann-Arbor stage IV and 5 (28%) had bone marrow infiltration. Median lymphoma cells proliferation fraction assessed by Ki-67 IHC was 90% (range 70-100%). Patients received a median of 6 courses (range 2-6) of the GMALL schedule, as inpatient therapy. No patients died of infectious complications during chemotherapy. Twelve patients (67%) were in complete metabolic response (CR) at end of treatment and another patient in complete response by CT scan. Of the 5 refractory patients, four have died (3 for lymphoma progression, one due to infectious complications after salvage splenectomy plus RT), one is still alive, under salvage therapy at 7 months from end of treatment. Among 12 patients who achieved CR, 3 relapsed at 7, 10, 16 months from end of treatment, they are presently alive under salvage treatment. At a median follow up of 21.7 month, the median PFS is 21.4 months while median OS is not reached. The two-year PFS and OS rates are 42% and 66%, respectively (figure 1 A & B). PFS was significantly worse in patients with bone marrow infiltration with median PFS of 7 months while not reached in the rest of patients. The median PFS was approximately 17 months for patients older than 55 years (n=13) and not reached for younger patients, with PFS at 2 years of 75%. There was no correlation with transformation, or Ki-67.
Conclusion: This study indicates that intensive treatment with B-ALL/NHL 2002 GMALL protocol is effective and well tolerated in true and atypical DHL patients aged up to 77 years. In this preliminary series, the OS resulted superior to that commonly reported with standard chemo-immunotherapies, with median 17-month OS in older patients while median OS is not reached in younger patients. In fact, the results were particularly promising in patients younger than 55 years probably reflecting the proper dose schedule delivery in younger patients. Based on these preliminary results, we are continuing to use the GMALL protocol as upfront therapy in DH lymphoma patients and we are exploring the possibility of improving the therapeutic efficacy by supplementing the GMALL schedule with one of the novel non-chemotherapeutic drugs.
Derenzini:TG-THERAPEUTICS: Research Funding. Saglio:Celgene: Consultancy; Jansen: Consultancy; Incyte: Consultancy; Pfizer: Consultancy; BMS: Consultancy; Novartis: Consultancy; Ariad: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.