Introduction:T cell engaging bispecific antibodies (T-BsAb) redirect cytotoxic T cells to tumor associated antigen (TAA)-positive target cells, leading to T-cell activation and lysis of target cells. Blinatumomab is the only T-BsAb approved by FDA. Numerous T-BsAb are undergoing clinical trials, targeting both hematological malignancies (e.g. BCMA, CD123 and CD33) and solid tumors (e.g. CEA, EpCAM, and GPC3). While these T-BsAbs represent a promising novel therapeutic approach, their efficacy remains modest. Ibrutinib is an irreversible inhibitor of Bruton's tyrosine kinase (BTK) and induces durable remissions in chronic lymphocytic leukemia (CLL). Our group has reported that ibrutinib has favorable immunomodulatory effects due to inhibition of interleukin-2 inducible T-cell kinase (ITK). Ibrutinib decreased key immunosuppressive checkpoint molecules and the frequency of regulatory T cells (Treg)(Long, Beckwith et al. 2017). Moreover, Ibrutinib significantly increased the number of the activated T cells by rescuing them from activation induced cell death (AICD). However, the function of ibrutinib 'rescued' T cells and their ability to mediate T-BsAb redirected cytotoxicity is unknown. Therefore we proceeded to study how ibrutinib treatment affects the functional competency of T cells by measuring their capability to mediate Blinatumomab redirected cytotoxicity.
To examine T cell function post ibrutinib treatment, T cells were isolated from pre and post treatment PBMC samples by fluorescence activated cell sorting (FACS), and used as effector cells with pre-treatment CLL B-cells as target cells. Blinatumomab redirected T-cell cytotoxicity against tumor cells and T cell survival were assayed by flow cytometry based methods. Effector cells (T cells) were labeled with CFSE. Target (CLL) cells were labeled with CellTrace Violet. They were co-cultured at an E:T ratio of 4:1 with Blinatumomab for 24 hours. Effector cells and target cells can be distinguished by different fluorescence labels. Apoptosis and cell death were evaluated by Annexin-V and PI staining. The number of viable target cells and effector cells were calculated by Countbright counting bead.
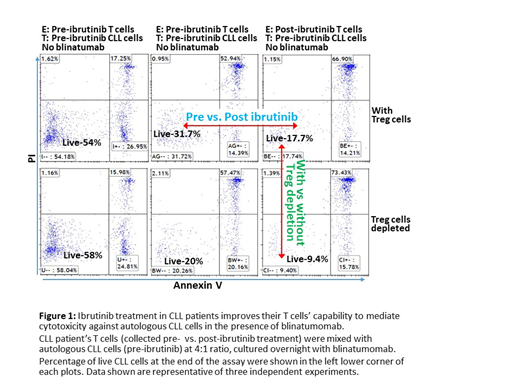
Results: We observed that post-ibrutinib T cells demonstrated significantly superior cytotoxicity against autologous CLL cells in the presence of blinatumomab in three independent patient samples. Numbers of viable CLL cells co-cultured with post-ibrutinib T cells were lower when compared to those co-cultured with pre-ibrutinib T cells (33.6%, 68% and 55.1% reduction in viable CLL cells for the three patient samples, respectively). We also found that Treg depletion (by depleting CD25high/CD127low CD4+ T cells) further enhanced cytotoxicity for both pre and post-ibrutinib treatment T cells, with the numbers of viable CLL cells reduced another 30-40%. Moreover, compared to pre-ibrutinib T cells, post-ibrutinib treatment T cells again demonstrated superior cytotoxicity when Treg cells were depleted, with decreased numbers of viable CLL cells at the end of co-culture (43%, 69% and 49.4% reduction, respectively). We also observed that the numbers of viable T cells from pre-ibrutinib samples were much lower than post-ibrutinib samples (52.5%, 77.9% and 74% reduction in viable T cells, respectively).
Conclusions: Our findings suggest that ibrutinib-rescued T cells are functionally competent. CLL patients' T cells post ibrutinib treatment demonstrated superior cytotoxicity against autologous leukemia cells compared to T cells from treatment baseline. Moreover, we show that increased T cell activity is not solely due to ibrutinib-related Treg depletion. Treg depletion in our experiments further enhanced the cytotoxicity of both post and pre-ibrutinib treatment T cells while post-ibrutinib T cells still demonstrated superior cytotoxicity (Fig 1). Lastly, T cells isolated from post-ibrutinib samples demonstrated significantly improved viability after in-vitro stimulation with blinatumomab, which is likely a result of reduced AICD.
Bhat:Pharmacyclics: Consultancy; Janssen: Consultancy. Rogers:Janssen: Research Funding; AbbVie: Research Funding; Genentech: Research Funding; Acerta Pharma: Consultancy. Woyach:Janssen: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; AbbVie: Research Funding; Karyopharm: Research Funding; Loxo: Research Funding; Morphosys: Research Funding; Verastem: Research Funding. Muthusamy:Ohio State University: Patents & Royalties: OSU-2S. Byrd:Novartis: Other: Travel Expenses, Speakers Bureau; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; BeiGene: Research Funding; Ohio State University: Patents & Royalties: OSU-2S; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Acerta: Research Funding; Genentech: Research Funding; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Acerta: Research Funding; Ohio State University: Patents & Royalties: OSU-2S; BeiGene: Research Funding; Novartis: Other: Travel Expenses, Speakers Bureau; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; Genentech: Research Funding; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.